
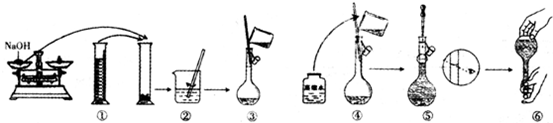
| n |
| V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
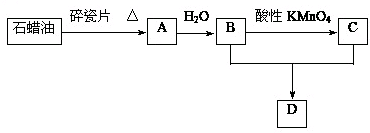
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ᴿʱ��Ϊ�˼ӿ�������ʣ����ò���������������е�Һ�� |
| B����������ƽ��ȡ10.5 g NaClʱӦ���ұ������з���10 g���� |
| C����Һ����ʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| D���ⶨ��ҺpH�IJ�����pH��ֽ���ڱ������ϣ��ò�����պȡ��Һ������pH��ֽ���в���Ȼ�������ɫ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
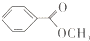 ����һ����Ҫ�Ĺ�ҵ�л��ܼ�������������л���Ľṹ��ʽ�������ص㣬Ȼ��ش����⣮
����һ����Ҫ�Ĺ�ҵ�л��ܼ�������������л���Ľṹ��ʽ�������ص㣬Ȼ��ش����⣮
| ���ķ���ʽ | C7H8O |
| ���IJ������� | ����״̬�������Ʒ�Ӧ�ų����� |
| ��FeCl3��Һ��������ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ȼҵ��ͨ����ⱥ��ʳ��ˮ�����������ռ� |
| B��ͨ��״���£�����������ܺ�Fe��Ӧ |
| C��������H2��Cl2�а�����ȼ�գ�������ɫ���棬����ƿ�ڳ�����״������H2��Cl2�Ļ������ʱ����Ѹ�ٻ��϶���ը |
| D��������ˮ����Ư���ԣ�������ˮ���ձ�Ϊϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��������� | �����Լ��� | ||
| ����1 | ����2 | ||
| A | ľ̿�ۺ�����ͭ | �۲���ɫ | ͨCO������ |
| B | NaCl��Һ��Na2CO3��Һ | ϡHCl | Zn |
| C | NH4HCO3���ʺ�K2SO4�ط� | ��NaOH��ĥ������ζ | ϡH2SO4 |
| D | ϡHCl��KCl��Һ | Fe2O3 | ��ɫ��̪ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K=
| ||
| B����ʱ��B��ƽ��ת������35% | ||
| C���������ϵ��ѹǿ��ƽ�������ƶ�����ѧƽ�ⳣ������ | ||
| D������C��B��ƽ��ת���ʲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com