
| ʵ�鲽�� | Ԥ�������� |
| ����1��ȡ������Ʒ���Թ��У��μ�������1mol?L-1H2SO4��Һ�������ܽ⣬�õ�A��Һ | |
| ����2�� | �����������Һ�Ϻ�ɫ��ȥ������Ʒ�к�+2�۵���Ԫ�� |
| ����3�� | ����Һ���ɫ������Ʒ�к�+3�۵���Ԫ�� |
���� ��1����������������Ԫ�صļ�̬Ϊ+2��+3����+2��+3��
�ڼ����������ӣ��ø��������Һ�������������ɫ��˵�������������ӣ�������������KSCN��Һ�������ɫ˵�����������ӣ�
��ϡ�������ǿ�����ԣ��������������ܽ⣻
��2����KMnO4��Һ����ɫ������������ָʾ����
�ڸ��ݷ�Ӧ5Fe2++MnO4-+8H+�T5Fe3++Mn2++4H2O���ɵó�5Fe2+��MnO4-������ MnO4-��Һ���Ϊ21.50mLʱ�������Ӧ���������ӵ����ʵ����������250mL��Һ���������ӵ����ʵ�������������������������
��� �⣺��1����������������Ԫ�صļ�̬Ϊ+2��+3����+2��+3����������������ʯ����Ԫ�صļ�̬Ϊ+2��+3���ʴ�Ϊ��������ʯ����Ԫ�صļ�̬Ϊ+2��+3��
�ڼ����������ӣ��ø��������Һ����ȡ2mLA��Һ���Թ��У��ý�ͷ�ιܵμ�1��2��0.01mol•L-1KMnO4��Һ�����Թܣ������������ɫ��˵�������������ӣ�������������KSCN��Һ��ȡ2mLA��Һ���Թ��У��ý�ͷ�ιܵμ�1��2��20%KSCN��Һ�����Թܣ�����Һ���ɫ˵�����������ӣ��ʴ�Ϊ��
| ʵ�鲽�� | Ԥ�������� |
| ����2��ȡ��������1�����õ�A��Һ���Թ��У��μӼ���0.01mol?L-1���������Һ���� | ���������Һ�Ϻ�ɫ��ȥ |
| ����3��ȡ��������1�����õ�A��Һ���Թ��У��μӼ���0.01mol?L-1KSCN��Һ���� | ��Һ���ɫ |
���� ���⿼����ʵ����ƵĻ���ԭ�������ӵļ��鷽�������ʺ����IJⶨʵ�鷽����ƣ���Ŀ�Ѷ��еȣ������ڿ���ѧ����ʵ��̽�������ͼ���������ע����ճ������ӵļ��鷽����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ClO3-��Cl-��K+ | B�� | ClO-��Cl-��H+ | ||
| C�� | NaClO��NaClO3��NaNO3 | D�� | NaClO��Na2SO4��NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢۢܢ�� | B�� | �ݢߢ��� | C�� | �ڢۢܢݢ� | D�� | �٢ޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��1����2����4����5����6�� | B�� | ��1����3����4����5����6�� | C�� | ��1����2����3����5����6�� | D�� | ֻ�У�1����3����5�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���ڱ���ǰ������Ԫ��R��W��X��Y��Z ��ԭ���������ε�����R�Ļ�̬ԭ����ռ��������ԭ�ӹ���ĵ�����Ϊ1��W���⻯��ķе��ͬ������Ԫ���⻯��ķе�ߣ�X2+��W2-������ͬ�ĵ��Ӳ�ṹ��YԪ��ԭ�ӵ�3P�ܼ����ڰ����״̬��Z+�ĵ��Ӳ㶼�������ӣ���ش��������⣺
���ڱ���ǰ������Ԫ��R��W��X��Y��Z ��ԭ���������ε�����R�Ļ�̬ԭ����ռ��������ԭ�ӹ���ĵ�����Ϊ1��W���⻯��ķе��ͬ������Ԫ���⻯��ķе�ߣ�X2+��W2-������ͬ�ĵ��Ӳ�ṹ��YԪ��ԭ�ӵ�3P�ܼ����ڰ����״̬��Z+�ĵ��Ӳ㶼�������ӣ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
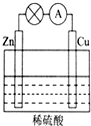 ��1����ͼ��ʾ��ԭ���װ���У��为��������Zn���������ܹ��۲쵽��������ͭƬ���������ɫ���ݣ������ĵ缫��Ӧʽ��2H++2e-=H2����ԭ��ع���һ��ʱ���������п6.5g����ų�����0.2g��
��1����ͼ��ʾ��ԭ���װ���У��为��������Zn���������ܹ��۲쵽��������ͭƬ���������ɫ���ݣ������ĵ缫��Ӧʽ��2H++2e-=H2����ԭ��ع���һ��ʱ���������п6.5g����ų�����0.2g���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com