
| |||||||||||||||||||||||||||||||||||||
 ŌĘÄĻŹ¦“óø½Š”Ņ»ĻßĆūŹ¦ĢįÓÅ×÷ŅµĻµĮŠ“š°ø
ŌĘÄĻŹ¦“óø½Š”Ņ»ĻßĆūŹ¦ĢįÓÅ×÷ŅµĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| øßĪĀ |
| c3(H2O) |
| c3(H2) |
| c3(H2O) |
| c3(H2) |
| 1400”ę |
| Ō¼3000”ę |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| øßĪĀ |
| c3(H2O) |
| c3(H2) |
| c3(H2O) |
| c3(H2) |
| ĪĀ¶Č | 25”ꔫ550”ꔫ600”ꔫ700”ę |
| Ö÷ŅŖ³É·Ż | WO3 W2O5 WO2 W |
| ||
| ||
| ||
| Ō¼3000”ę |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012ÄźĘÕĶØøßµČѧŠ£ÕŠÉśČ«¹śĶ³Ņ»æ¼ŹŌĄķ×Ū»Æѧ²æ·Ö£ØĢģ½ņ¾ķ“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©½šŹōĪŁÓĆĶ¾¹ć·ŗ£¬Ö÷ŅŖÓĆÓŚÖĘŌģÓ²ÖŹ»ņÄĶøßĪĀµÄŗĻ½š£¬ŅŌ¼°µĘÅŻµÄµĘĖ攣øßĪĀĻĀ£¬ŌŚĆܱÕČŻĘ÷ÖŠÓĆH2»¹ŌWO3æɵƵ½½šŹōĪŁ£¬Ęä×Ü·“Ó¦ĪŖ£ŗ
WO3 (s) + 3H2 (g)  W (s) + 3H2O (g)
W (s) + 3H2O (g)
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Å ÉĻŹö·“Ó¦µÄ»ÆŃ§Ę½ŗā³£Źż±ķ“ļŹ½ĪŖ___________________________”£
¢Ę ijĪĀ¶ČĻĀ·“Ó¦“ļĘ½ŗāŹ±£¬H2ÓėĖ®ÕōĘųµÄĢå»ż±ČĪŖ2:3£¬ŌņH2µÄĘ½ŗā×Ŗ»ÆĀŹĪŖ_____________________£»ĖęĪĀ¶ČµÄÉżøߣ¬H2ÓėĖ®ÕōĘųµÄĢå»ż±Č¼õŠ”£¬ŌņøĆ·“Ó¦ĪŖ___________·“Ó¦£ØĢī”°ĪüČČ”±»ņ”°·ÅČČ”±£©”£
¢ĒÉĻŹö×Ü·“Ó¦¹ż³Ģ“óÖĀ·ÖĪŖČżøö½×¶Ī£¬ø÷½×¶ĪÖ÷ŅŖ³É·ÖÓėĪĀ¶ČµÄ¹ŲĻµČēĻĀ±ķĖłŹ¾£ŗ
| ĪĀ¶Č | 25”ę ~ 550”ę ~ 600”ę ~ 700”ę |
| Ö÷ŅŖ³É·Ż | WO3 W2O5 WO2 W |
 W (s) + 2H2O (g) ¦¤H£½ +66.0 kJ”¤mol£1
W (s) + 2H2O (g) ¦¤H£½ +66.0 kJ”¤mol£1 W (s) + 2H2O (g) ¦¤H £½ £137.9 kJ”¤mol£1
W (s) + 2H2O (g) ¦¤H £½ £137.9 kJ”¤mol£1 WO2 (g) µÄ¦¤H £½ ______________________”£
WO2 (g) µÄ¦¤H £½ ______________________”£ WI4 (g)”£ĻĀĮŠĖµ·ØÕżČ·µÄÓŠ________________”£
WI4 (g)”£ĻĀĮŠĖµ·ØÕżČ·µÄÓŠ________________”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ¹ć¶«Ź”ŹµŃé֊ѧø߶žÉĻѧʌʌ֊»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø16·Ö£©½šŹōĪŁÓĆĶ¾¹ć·ŗ£¬Ö÷ŅŖÓĆÓŚÖĘŌģÓ²ÖŹ»ņÄĶøßĪĀµÄŗĻ½š£¬ŅŌ¼°µĘÅŻµÄµĘĖ攣øßĪĀĻĀ£¬ŌŚĆܱÕČŻĘ÷ÖŠÓĆH2»¹ŌWO3æɵƵ½½šŹōĪŁ£¬Ęä×Ü·“Ó¦ĪŖ£ŗ
WO3£Øs£©+3 H2£Øg£© W£Øs£©+3 H2O£Øg£©
W£Øs£©+3 H2O£Øg£©
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÉĻŹö·“Ó¦µÄ»ÆŃ§Ę½ŗā³£Źż±ķ“ļŹ½ĪŖ
£Ø2£©Ä³ĪĀ¶ČĻĀ·“Ó¦“ļĘ½ŗāŹ±£¬ĖęĪĀ¶ČµÄÉżøߣ¬ H2ÓėĖ®ÕōĘųµÄĢå»ż±Č¼õŠ”£¬ŌņøĆ·“Ó¦ĪŖ_____________·“Ó¦(Ģī ĪüČČ»ņ·ÅČČ)
£Ø3£©ÉĻŹö×Ü·“Ó¦¹ż³Ģ“óÖĀ·ÖĪŖČżøö½×¶Ī£¬ø÷½×¶ĪÖ÷ŅŖ³É·ÖÓėĪĀ¶ČµÄ¹ŲĻµČēĻĀ±ķĖłŹ¾£ŗ
| ĪĀ¶Č | 25 ”ę ~ 550 ”ę ~ 600”ę ~ 700”ę |
| Ö÷ŅŖ³É·Ö | WO3 W2O5 WO2 W |
 W£Øs£©+2H2O£Øg£© ¦¤H£½+66 kJ·mol-1
W£Øs£©+2H2O£Øg£© ¦¤H£½+66 kJ·mol-1 W£Øs£©+2H2O£Øg£© ¦¤H£½ ”Ŗ137.9 kJ·mol-1
W£Øs£©+2H2O£Øg£© ¦¤H£½ ”Ŗ137.9 kJ·mol-1 WO2£Øg£©µÄ¦¤H£½_____ _______.
WO2£Øg£©µÄ¦¤H£½_____ _______.
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012ÄźĘÕĶØøßµČѧŠ£ÕŠÉśČ«¹śĶ³Ņ»æ¼ŹŌĄķ×Ū»Æѧ²æ·Ö£ØĢģ½ņ¾ķ½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©½šŹōĪŁÓĆĶ¾¹ć·ŗ£¬Ö÷ŅŖÓĆÓŚÖĘŌģÓ²ÖŹ»ņÄĶøßĪĀµÄŗĻ½š£¬ŅŌ¼°µĘÅŻµÄµĘĖ攣øßĪĀĻĀ£¬ŌŚĆܱÕČŻĘ÷ÖŠÓĆH2»¹ŌWO3æɵƵ½½šŹōĪŁ£¬Ęä×Ü·“Ó¦ĪŖ£ŗ
WO3 (s) + 3H2 (g)  W (s) + 3H2O (g)
W (s) + 3H2O (g)
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Å ÉĻŹö·“Ó¦µÄ»ÆŃ§Ę½ŗā³£Źż±ķ“ļŹ½ĪŖ___________________________”£
¢Ę ijĪĀ¶ČĻĀ·“Ó¦“ļĘ½ŗāŹ±£¬H2ÓėĖ®ÕōĘųµÄĢå»ż±ČĪŖ2:3£¬ŌņH2µÄĘ½ŗā×Ŗ»ÆĀŹĪŖ_____________________£»ĖęĪĀ¶ČµÄÉżøߣ¬H2ÓėĖ®ÕōĘųµÄĢå»ż±Č¼õŠ”£¬ŌņøĆ·“Ó¦ĪŖ___________·“Ó¦£ØĢī”°ĪüČČ”±»ņ”°·ÅČČ”±£©”£
¢ĒÉĻŹö×Ü·“Ó¦¹ż³Ģ“óÖĀ·ÖĪŖČżøö½×¶Ī£¬ø÷½×¶ĪÖ÷ŅŖ³É·ÖÓėĪĀ¶ČµÄ¹ŲĻµČēĻĀ±ķĖłŹ¾£ŗ
|
ĪĀ¶Č |
25”ę ~ 550”ę ~ 600”ę ~ 700”ę |
|
Ö÷ŅŖ³É·Ż |
WO3 W2O5 WO2 W |
µŚŅ»½×¶Ī·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ___________________________£»580”ꏱ£¬¹ĢĢåĪļÖŹµÄÖ÷ŅŖ³É·ÖĪŖ________£»¼ŁÉčWO3ĶźČ«×Ŗ»ÆĪŖW£¬ŌņČżøö½×¶ĪĻūŗÄH2ĪļÖŹµÄĮæÖ®±ČĪŖ____________________________________”£
¢Č ŅŃÖŖ£ŗĪĀ¶Č¹żøߏ±£¬WO2 (s)×Ŗ±äĪŖWO2 (g)£»
WO2 (s) + 2H2 (g)  W (s) + 2H2O (g)
¦¤H £½ +66.0 kJ”¤mol£1
W (s) + 2H2O (g)
¦¤H £½ +66.0 kJ”¤mol£1
WO2 (g) + 2H2(g)  W (s) + 2H2O (g)
¦¤H £½ £137.9 kJ”¤mol£1
W (s) + 2H2O (g)
¦¤H £½ £137.9 kJ”¤mol£1
ŌņWO2 (s)  WO2 (g) µÄ¦¤H £½
______________________ӣ
WO2 (g) µÄ¦¤H £½
______________________ӣ
¢É ĪŁĖæµĘ¹ÜÖŠµÄWŌŚŹ¹ÓĆ¹ż³ĢÖŠ»ŗĀż»Ó·¢£¬Ź¹µĘĖæ±äĻø£¬¼ÓČėI2æÉŃÓ³¤µĘ¹ÜµÄŹ¹ÓĆŹŁĆü£¬Ę乤×÷ŌĄķĪŖ£ŗW (s) +2I2 (g) 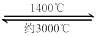 WI4 (g)”£ĻĀĮŠĖµ·ØÕżČ·µÄÓŠ________________”£
WI4 (g)”£ĻĀĮŠĖµ·ØÕżČ·µÄÓŠ________________”£
a£®µĘ¹ÜÄŚµÄI2æÉŃ»·Ź¹ÓĆ
b£®WI4ŌŚµĘĖæÉĻ·Ö½ā£¬²śÉśµÄWÓÖ³Į»żŌŚµĘĖæÉĻ
c£®WI4ŌŚµĘ¹Ü±ŚÉĻ·Ö½ā£¬Ź¹µĘ¹ÜµÄŹŁĆüŃÓ³¤
 d£®ĪĀ¶ČÉżøߏ±£¬WI4µÄ·Ö½āĖŁĀŹ¼Óæģ£¬WŗĶI2µÄ»ÆŗĻĖŁĀŹ¼õĀż
d£®ĪĀ¶ČÉżøߏ±£¬WI4µÄ·Ö½āĖŁĀŹ¼Óæģ£¬WŗĶI2µÄ»ÆŗĻĖŁĀŹ¼õĀż
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com