
| A����ˮ | B��ŨNaOH��Һ | C������ʯ��ˮ |
| D������KMnO4 E��Ʒ����Һ�������ʵ�鷽��֤�����Ƶõ���ϩ�л���CO2��SO2��ͬʱ��ϩ������ˮ��Ӧ�� |
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����25g CuSO4��5H2O����1Lˮ�У������Ƴ�0��1 mol��L-1CuSO4��Һ |
| B������������ȥ�������������л��е������� |
| C���������ſ������ռ�NH��������ʪ�����ɫʯ����ֽ����NH���Ƿ��ռ����� |
| D������Ȳʱ���ñ���ʳ��ˮ����ˮ��Ϊ�˼�����ʯ��ˮ�ķ�Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
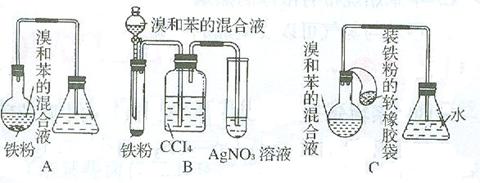
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
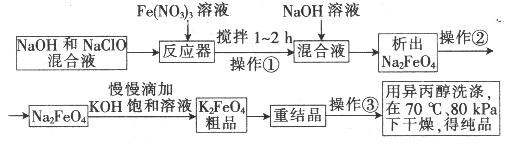
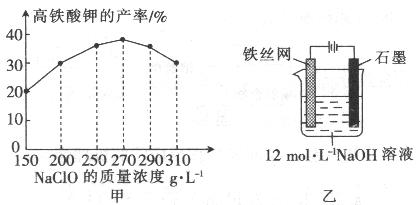
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
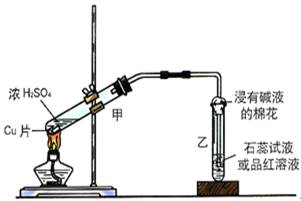
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


| A������Kͨ��N2���Թ�A�������ݲ�����˵��װ������������ |
| B��U�ι���ʢ�ŵĸ�����ȿ����ü�ʯ�ң�Ҳ������Ũ���� |
| C����Ӧ��������Ϩ��ƾ��ƣ�����Ӧ����ȴ���ٹرջ���K |
| D��������Ca3N2���������У�ֻ�ܵõ�һ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A����Cu��ϡ���ᷴӦ��NO |
| B����NaOH������Ũ��ˮ��Ӧ��NH3 |
| C����Fe��ϡ���ᷴӦ��H2 |
| D����MnO2��Ũ���ᷴӦ��Cl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������л�����ϩ��ͨ��������ʹ��ϩ�����ӳɷ�Ӧ |
| B���Ȼ�����Һ�л������������ƣ������������ᱵ��Һ������ |
| C���Ҵ��л������ᣬ�����������ƺ�Һ |
| D��������̼�����л��������Ķ�������ͨ�����Ը��������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
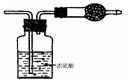
| A��������ڹ���Ϊ��ʯ�� | B��ԭ������һ���� NO �� O2 |
| C��ԭ������һ����NH3��NO ��CO2 | D��ԭ�������� HC1�� Br2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com