

(1)ʵ��ʱ����ⶨ��п�����������������֮�⣬�Ƿ���Ҫ����������������Ҫ����ָ��_______________________�����˿ղ��
(2)ʵ��ʱ�ڼ��װ�������Ժ���ν��Լ������Թܲ�ʹ��Ӧ��ʼ��_______________��
(3)�����Dz����ռ������������������ļ������裺�ٵ�����Ͳ����Һ��߶�ʹ֮��ͬ����ʹ�Թܺ���Ͳ�ڵ����嶼��ȴ�����£��۶�ȡ��Ͳ��������������������������ȷ˳���ǣ�_______________________������д������ţ���
(4)�����ռ������������ͼ�мг�������ʡ�ԣ�ʱ�����ʹ��Ͳ����Һ��ĸ߶���ͬ?______________________________________________��
(5)���ʵ���в�ô�п����Ϊa g���õ������������b L��������ɱ�״������ˮ������Ӱ����Բ��ƣ���п��п�����������ļ���ʽΪ����a��b�����ػ���___________________��
(6)�Ը��������������������ݵ�ʵ�鷽����_________________________________��
(1)�ⶨʵ��ʱ���¶ȡ�ѹǿ
(2)�����Թ��м�������ϡ���ᣬ�ٽ��Թ���б�������ӽ���п�����Թܿڸ��������õ�����Ƥ����Ȼ���Թܻ�������ʹ��п�����Թ��ڱڻ��������Թܵײ�
(3)�ڢ٢�
(4)��������Ͳ���ƣ�ʹ��Ͳ����Һ����ƽ
(5)b L��22.4 L��mol-1��65 g��mol-1��a g��100%
(6)ʹп������ϡ�����ַ�Ӧ���˳�ʣ����壬ϴ�ӡ����������Ӧǰ����������
�����������dz����½���ʵ�飬�����ʵ��ʱ��Ũ�ȡ�ѹǿ���Ա㽫H2�������Ϊ��״���µ��������������Լ�Ӧ���չ涨�ķ������룬�������Թܡ����ڲ��������ʱ����ʹ����ѹǿ��ȣ����������Ͳ�߶ȣ�ʹ���е�Һ�����Թ�Һ��ˮƽ�������������ݣ�Zn�Ĵ���Ϊ��![]() ��65 g��mol-1��a g��100%��
��65 g��mol-1��a g��100%��
�������ʲ����ᷴӦ���ʿ��ó���ʣ����������ķ�������Zn�Ĵ��ȡ�


 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
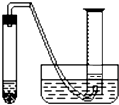 ijѧ����ͨ���ⶨ��п��������ϡ�����Ӧ����ϡ���ᷴӦ������������������ⶨ��п��п�Ĵ��ȣ����д�п��ϡ���ᡢ�Թܡ����ӡ�������Ƥ���������ܡ�������ƽ����Ͳ��ˮ�ۡ�����̨�������У���������ҩƷ��
ijѧ����ͨ���ⶨ��п��������ϡ�����Ӧ����ϡ���ᷴӦ������������������ⶨ��п��п�Ĵ��ȣ����д�п��ϡ���ᡢ�Թܡ����ӡ�������Ƥ���������ܡ�������ƽ����Ͳ��ˮ�ۡ�����̨�������У���������ҩƷ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007��������Զ��ѧ������ѧ(������)�¿��Ծ� ���ͣ�058
| |||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫������ѧ2007�����ڶ��ֶ���ʵ��ר����ϰ����ѧ ���ͣ�058
| |||||||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ����ͨ���ⶨ��п��������ϡ�����Ӧ����ϡ���ᷴӦ������������������ⶨ��п��п�Ĵ��ȡ����ô�п��ϡ���ᡢ�Թܡ����ӡ�������Ƥ���������ܡ�������ƽ����Ͳ��ˮ�ۡ�����̨�������У���������ҩƷ����ʵ�顣ʵ��װ����ͼ��ʾ���̶�װ��δ��������

��1���÷�Ӧ�����ӷ���ʽΪ ��
��2��ijѧ������11.9 mol/L Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0��400 mol��L��ϡ���ᡣ��ѧ����Ҫ����Ͳ��ȡ mL����Ũ����������ơ�
��3��ʵ��ʱ����ⶨ��п�����������������֮�⣬�Ƿ���Ҫ����������������Ҫ����ָ��____________________�����˿ղ��
��4���ڼ���ҩƷ֮ǰ����װ�������ԣ���μ���װ�õ������ԣ�
��
��5�������Dz����ռ������������������ļ������裺
�ٵ�����Ͳ����Һ��߶�ʹ֮��ͬ��
��ʹ�Թܺ���Ͳ�ڵ����嶼��ȴ�����£�
�۶�ȡ��Ͳ������������
��������������ȷ˳���ǣ�________________________������д������ţ���
��6�����ʵ���в�ô�п����Ϊa g���õ������������b L��������ɱ�״������ˮ������Ӱ����Բ��ƣ���п��п�����������ļ���ʽΪ���ú�a��b�Ĵ���ʽ��ʾ�����ػ���___________________��
��7���������һ�ָ���ݵ�ʵ�鷽����________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com