
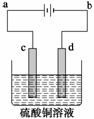
(1)[2012���¿α�ȫ������36(3)]��ͭ�ĵ�⾫����ͼ��ʾ���ڴ�ͭ�ĵ������У���ͭ��Ӧ��ͼ�е缫________(��ͼ�е���ĸ)���ڵ缫d�Ϸ����ĵ缫��ӦʽΪ__________������ͭ�л�����Au��Ag��Fe�������ڵ����еĴ�����ʽ��λ��Ϊ____________��
(2)(2012�����ϣ�16)����Ч�ļ���ȼ�ϵ�ز��ò�Ϊ�缫���ϣ����缫�Ϸֱ�ͨ��CH4��O2 �������ΪKOH��Һ��ij�о�С�齫��������ȼ�ϵ�ش�������Ϊ��Դ�����б����Ȼ�����Һ���ʵ�飬��ͼ��ʾ��
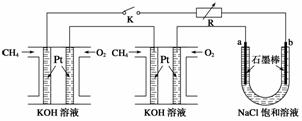
�ش��������⣺
�ټ���ȼ�ϵ�������������ĵ缫��Ӧ�ֱ�Ϊ________________________________��
________________________________________________________________________��
�ڱպ�K���غ�a��b�缫�Ͼ����������������b�缫�ϵõ�����__________������Ȼ�����Һ���ܷ�Ӧ����ʽΪ
________________________________________________________________________��
����ÿ����ؼ���ͨ����Ϊ1 L(��״��)���ҷ�Ӧ��ȫ����������ͨ�����صĵ���Ϊ__________(�����ڳ���F��9.65��104 C �� mol��1��ʽ����)������ܲ������������Ϊ________L(��״��)��
�𰸡�(1)c��Cu2����2e��===Cu��Au��Ag�Ե��ʵ���ʽ������c(����)�·���Fe��Fe2������ʽ������Һ
(2)��O2��2H2O��4e��===4OH��
CH4��10OH����8e��===CO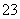 ��7H2O
��7H2O
��H2��2NaCl��2H2O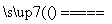 2NaOH��H2����Cl2��
2NaOH��H2����Cl2��
�� ��8��9.65��104 C��mol��1
��8��9.65��104 C��mol��1
��3.45��104 C��4
������(1)��⾫��ʱ����ͭ����������ͭ��������CuSO4��Һ�����Һ����ͭ���õĽ���ʧ�����������ӽ�����Һ������ͭ���õĽ����γ����������������
(2)�ټ���ȼ�ϵ��������ΪO2����ԭ������ΪCH4���������ڼ�������������CO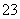 ����缫��Ӧ�ֱ�ΪO2��2H2O��4e��===4OH����CH4��10OH����8e��===CO
����缫��Ӧ�ֱ�ΪO2��2H2O��4e��===4OH����CH4��10OH����8e��===CO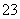 ��7H2O��
��7H2O��
�ڱպ�K�ɱպϻ�·����ʱa��b�ֱ�Ϊ���ص�����������������ΪH���õ��ӱ���ԭ��ͬʱ�õ�H2��NaOH������ΪCl���������õ�Cl2���õ����е��ܷ�ӦΪ2NaCl��2H2O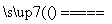 2NaOH��H2����Cl2����
2NaOH��H2����Cl2����
��ÿ�����ͨ��1 L CH4����·��ͨ���ĵ���Ϊ ��8��9.65��104 C��mol��1��3.45��104 C��
��8��9.65��104 C��mol��1��3.45��104 C��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ����д�缫��Ӧʽ���ܷ���ʽ
(1)�ö��Ե缫���AgNO3��Һ��
������Ӧʽ_______________________________________________________________��
������Ӧʽ_______________________________________________________________��
�ܷ�Ӧ���ӷ���ʽ_________________________________________________________��
(2)�ö��Ե缫���MgCl2��Һ
������Ӧʽ_______________________________________________________________��
������Ӧʽ_______________________________________________________________��
�ܷ�Ӧ���ӷ���ʽ_________________________________________________________��
(3)�������缫���NaCl��Һ
������Ӧʽ_______________________________________________________________��
������Ӧʽ_______________________________________________________________��
�ܻ�ѧ����ʽ_____________________________________________________________��
(4)��ͭ���缫���������Һ
������Ӧʽ_______________________________________________________________��
������Ӧʽ_______________________________________________________________��
�ܷ�Ӧ���ӷ���ʽ_________________________________________________________��
(5)��Al���缫���NaOH��Һ
������Ӧʽ_______________________________________________________________��
������Ӧʽ_______________________________________________________________��
�ܷ�Ӧ���ӷ���ʽ_________________________________________________________��
(6)�ö��Ե缫�������MgCl2
������Ӧʽ_______________________________________________________________��
������Ӧʽ_______________________________________________________________��
�ܷ�Ӧ���ӷ���ʽ________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ʴ�йص�˵������ȷ���� (����)


A��ͼ1�� �������ױ���ʴ
�������ױ���ʴ
B��ͼ2�У��μ�����K3[Fe(CN)6]��Һ��û����ɫ��������
C��ͼ3�У�ȼ��������IJ�λ�������⣬��Ҫ�����ڸ�������������ѧ��ʴ
D��ͼ4�У�������þ��ķ�������ֹ���¸����ܵ��ĸ�ʴ��þ���൱��ԭ��ص�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��ǿ������ʴ�ԣ�����Ǧ����Ϊ���Դ����Al��������Pb�����������ϡ���ᣬʹ�����������Ĥ����Ӧԭ�����£�
��أ�Pb(s)��PbO2(s)��2H2SO4(aq)= ==2PbSO4(s)��2H2O(l)
==2PbSO4(s)��2H2O(l)
���أ�2Al��3H2O���,Al2O3��3H2��
�������У������ж���ȷ���� (����)
| ��� | ���� | |
| A | H������Pb�缫 | H������Pb�缫 |
| B | ÿ����3 mol Pb | ����2 mol Al2O3 |
| C | ������PbO2��4H����2e��===Pb2����2H2O | ������2Al��3H2O��6e��===Al2O3��6H�� |
| D |
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
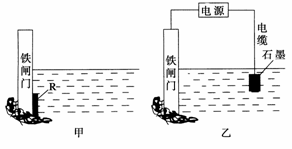
�������������������ʴ��ÿ����ʴ����ʧ�ĸ���ռ���������������ķ�֮һ��
(1)������ʴ��Ҫ��������ʴ���ø�ʴ�����еĵ缫��ӦʽΪ___________________��
(2)Ϊ�˽���ijˮ�����բ�ű���ʴ�����ʣ����Բ���ͼ����ʾ�ķ��������к�������բ ���ϵĹ������R���Բ���________(��д��ĸ���)��
A��ͭ B���� C��п D��ʯī
(3)ͼ����ʾ�ķ���Ҳ���Խ�����բ�ŵĸ�ʴ���ʣ�������բ��Ӧ��������ֱ����Դ��________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������кȹ��ף�ƻ������һ����ƻ�����Ͷ��ɵľ��нⶾ����֬�����ʺ�ֹк������ҩЧ�Ľ���ʳƷ��ƻ����(  )���������ϵ���Ҫ�������ʡ��������˵������ȷ����( )
)���������ϵ���Ҫ�������ʡ��������˵������ȷ����( )
A��ƻ������һ���������ܷ���������Ӧ
B��ƻ������һ���������ܷ�����������Ӧ
C��ƻ������һ���������ܷ�����ȥ��Ӧ
D��1 molƻ������Na2CO3��Һ��Ӧ����������2 mol Na2CO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ݱ�������һ�ֽ�Thibacillus Ferroxidans��ϸ�������������µ�������Һ�У��ܽ���ͭ��(CuFeS2)�����������Σ������ķ�ӦΪ��
4CuFeS2��2H2SO4��17O2===4CuSO4��2Fe2(SO4)3��2H2O
(1)CuFeS2��Fe�Ļ��ϼ�Ϊ��2��������Ӧ�б�������Ԫ����________��
(2)��ҵ����������������Ӧ�����Һ�����������̿��Ʊ�����(CuSO4��5H2O)��

�ٷ������б���(����Ksp����Ӧ������������ij����ܽ�ƽ�ⳣ��)��
| Ksp | �������↑ʼ ����ʱ��pH | ����������� ��ȫʱ��pH | |
| Fe3�� | 2.6��10��39 | 1.9 | 3.2 |
| Cu2�� | 2.2��10��20 | 4.7 | 6.7 |
����һӦ������Һ��pH��Χ��__________�������ó����ܽ�ƽ����й����۽��ͼ���CuO�ܳ�ȥCuSO4��Һ��Fe3����ԭ��________________________________________________
________________________________________________________________________��
�ڲ������еľ������������_________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com