
�����������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3��5H2O)��
��.���������ϡ�
(1)Na2S2O3��5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
(2)��Na2CO3��Na2S�����Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ����������Na2SO3��Na2SO4��
(3)Na2SO3�ױ�������BaSO3������ˮ��������ϡ���ᡣ
��.���Ʊ���Ʒ��
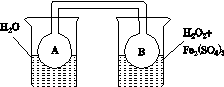
ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)��
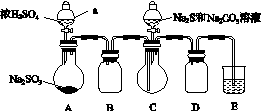
ʵ�鲽�裺
(1)���װ�������ԣ���ͼʾ�����Լ���
����a��������________��E�е��Լ���________(ѡ��������ĸ���)��
A��ϡH2SO4
B��NaOH��Һ
C������NaHSO3��Һ
(2)����C����ƿ����Na2S��Na2CO3�Ļ����Һ������A����ƿ�μ�ŨH2SO4��
(3)��Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�еĻ�����Һ��________(��д��������)���ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��.��̽���뷴˼��
(1)Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4����С�����������ʵ�鷽�����뽫��������������(�����Լ���ϡHNO3��ϡH2SO4��ϡ���ᡢ����ˮ��ѡ��)
ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ���а�ɫ�������ɣ�____________________________________��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
(2)Ϊ����װ��C������Na2SO4�������ڲ��ı�ԭ��װ�õĻ����϶�ʵ�鲽��(2)�����˸Ľ����Ľ���IJ�����______________________________________________________________________________��
(3)Na2S2O3��5H2O���ܽ�����¶����������������ò�Ʒͨ��________�����ᴿ��
��.(1)�ٷ�Һ©������B
(3)����
��.(1)���ˣ�������ˮϴ�ӳ�����������м�������ϡ���ᡡ(2)����A����ƿ�μ�ŨH2SO4�����������彫װ���еĿ����ž�������C����ƿ����Na2S��Na2CO3�����Һ
(3)�ؽᾧ
[����] ��.(1)��aΪ��Һ©������E���Լ���������SO2β������ΪNaOH��Һ��(3)����Na2S2O3��5H2O����ɫ�����壬������ˮ����Ҫ����Һ�еõ���������ƾ��壬�辭������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ�����õ���Ʒ����. (1)Na2S2O3��5H2O��ϡ��Һ��BaCl2����������ɣ���ʵ��������а�ɫ�������ɣ������һ����֤�������ɫ�����еμ�ϡ���ᣬ������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��(2)Na2SO3�����ױ������е�����������Na2SO4��Ϊ�˼���Na2SO4���ɵ��������ſ�װ���еĿ�����(3)Na2S2O3��5H2O���ܽ�����¶������������ᾧʱ���������������ʣ����ͨ���ؽᾧ�ķ����ᴿ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������е�̼ԭ��������ԭ�ӵĽ�Ϸ�ʽ��
A���γ��ĶԹ��õ��Ӷ� B��ͨ���Ǽ��Լ�
C��ͨ���������ۼ� D��ͨ�����Ӽ����ۼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊʵ������ʵ��Ŀ�ģ������±��ṩ����Ҫ�����������Լ���������(����)
| ѡ�� | ʵ��Ŀ�� | ��Ҫ���� | �Լ� |
| A | ����Br2��CCl4�Ļ���� | ��Һ©�����ձ� | Br2��CCl4�Ļ�������ˮ |
| B | ���������Ǻ����� | �Թܡ��ձ����ƾ��� | ��������Һ��������Һ��������Һ |
| C | ʵ������ȡH2 | �Թܡ������ܵ���Ƥ�� | п����ϡHNO3 |
| D | �ⶨNaOH��Һ��Ũ�� | �ζ��ܡ���ƿ���ձ� | NaOH��Һ�� 0��100 0 mol��L��1���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҷ����Ԫ�صļ���ɾ������ĸ�������ɣ���������ѡ�õ�ʵ����Ʒ���ܶ��õ�����(����)
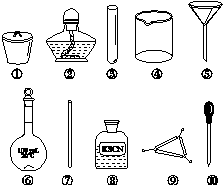
A������Ҷ���ջһ���ѡ�â١��ں͢�
B����Ũ�����ܽ��Ҷ��������ˮϡ�ͣ�ѡ�âܡ��͢�
C�����˵õ���Һ��ѡ�âܡ��ݺ͢�
D��������Һ�е�Fe3����ѡ�âۡ���͢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
H2O2��һ����ɫ������ԭ�Լ����ڻ�ѧ�о���Ӧ�ù㷺��
(1)ijС������ͬŨ��Fe3���Ĵ��£�̽��H2O2Ũ�ȶ�H2O2�ֽⷴӦ���ʵ�Ӱ�졣��ѡ�Լ���������30%H2O2��Һ��0.1 mol��L��1Fe2(SO4)3��Һ������ˮ����ƿ��˫������ˮ�ۡ����ܡ��������ܡ���Ͳ�����������ˮԡ�ۡ�ע������
��д����ʵ��H2O2�ֽⷴӦ����ʽ����������ת�Ƶķ������Ŀ��______________________________��
�����ʵ�鷽�����ڲ�ͬH2O2Ũ���£��ⶨ________(Ҫ������õ�������ֱ�����ַ�Ӧ���ʴ�С)��
�����ʵ��װ�ã����ͼ�е�װ��ʾ��ͼ��
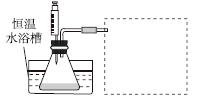
�ܲ����±���ʽ���ⶨʵ�������������ʵ�鷽��(�г���ѡ�Լ���������¼�Ĵ��������������ⶨ�����ݣ���������ĸ��ʾ)��
| ���������� ʵ����� ���� | V[0.1 mol��L��1 Fe2(SO4)3]/mL | ���� | |
| 1 | a | ���� | |
| 2 | a | ���� |
(2)����ͼ(a)��(b)�е���Ϣ����ͼ(c)װ��(��ͨ��A��Bƿ���ѳ���NO2����)����ʵ�顣�ɹ۲쵽Bƿ��������ɫ��Aƿ�е�__________(����dz��)����ԭ����____________________________��
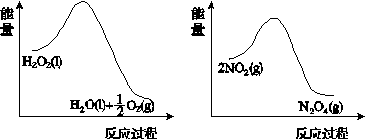
����������(a)������������������������(b)
(c)
ͼ21
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ҴӺ����Һ(��H2O�⣬����CCl4��I2��I����)�л��յ⣬��ʵ��������£�

(1)���Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI���������ӷ���ʽΪ__________________���ò�����I2��ԭΪI����Ŀ����______________________��
(2)����X������Ϊ________��
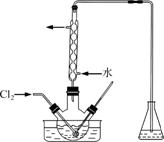
(3)����ʱ����������ƿ�н���I����ˮ��Һ���������pHԼΪ2������ͨ��Cl2����40 �����ҷ�Ӧ(ʵ��װ����ͼ��ʾ)��
ʵ������ڽϵ��¶��½��е�ԭ����______________����ƿ��ʢ�ŵ���ҺΪ________��
(4)��֪��5SO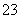 ��2IO
��2IO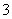 ��2H��===I2��5SO
��2H��===I2��5SO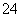 ��H2O
��H2O
ij�����ˮ(pHԼΪ8)��һ������I2�����ܴ���I����IO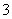 �е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO
�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO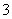 ��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼK381�ǡ�MnO2��H2O2�ֽⷴӦ����Ӱ����о���������ʾ��ͼ�������й�˵������ȷ����(����)
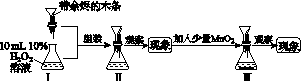
ͼK381
A��ʵ��ʱ�ȼ�H2O2��Һ���MnO2������������̡�Һ�ĽӴ����
B��Ϊʹʵ��˳�����У�H2O2��Һ���˴�ͼ��©��������
C�����ɹ۲쵽Ѹ�ٲ����������ݣ��������ľ����ȼ
D�����������в����ȼ���MnO2���ٲ���������ľ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���в���ȷ���� ( )
A������з�̪��ˮ�м������Na202��ĩ������Һ����ɫ����ֱ���ɫ
B�������Na202��ĩ����֬���ϵμӼ���ˮ����֬����ȼ��������˵��Na202��H20��Ӧ��һ�����ȷ�Ӧ������������
C��Na202��H20��Ӧ��һ��������ԭ��Ӧ��Na202���������������ǻ�ԭ��
D��Na202��H20��Ӧ��һ���û���Ӧ���е���02����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A��������ֱ���������Է�����3��̼ԭ��Ҳ��һ��ֱ����
B����ϩ����ԭ�Ӿ���ͬһƽ����
C��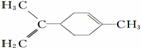 ����̼ԭ��һ����ͬһƽ����
����̼ԭ��һ����ͬһƽ����
D�� ������16��ԭ�ӹ�ƽ��
������16��ԭ�ӹ�ƽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com