
£®7∑÷£©œ¬±Ì «‘™Àÿ÷Ð∆⁄±Ìµƒ“ª≤ø∑÷£¨±Ì÷–À˘¡–µƒ◊÷ƒ∏∑÷±¥˙±Ì“ª÷÷ªØ—ß‘™Àÿ°£
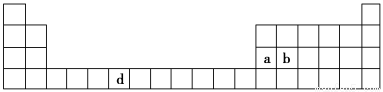
(1)«Î–¥≥ˆ‘™Àÿdµƒª˘Ã¨‘≠◊”µÁ◊”≈≈≤º Ω________________________________________°£
(2)b‘™Àÿµƒ—ıªØŒÔ÷–b”Ηı‘™Àÿ÷ƺ‰µƒπ≤º€º¸¿ý–Õ «________°£∆‰÷–b‘≠◊”µƒ‘”ªØ∑Ω Ω «_______°£
(3)aµ•÷ æßÃÂ÷–‘≠◊”µƒ∂—ª˝∑Ω Ω»Áœ¬Õºº◊À˘ 棨∆‰æß∞˚Ãÿ’˜»Áœ¬Õº““À˘ 棨‘≠◊”÷ƺ‰œýª•Œª÷√πÿœµµƒ∆Ω√ÊÕº»Áœ¬Õº±˚À˘ æ°£
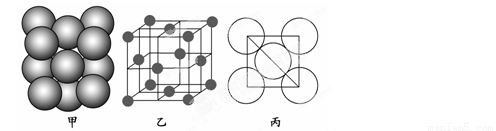
»Ù“—÷™aµƒ‘≠◊”∞Îæ∂Œ™d£¨NA¥˙±Ì∞¢∑¸º”µ¬¬Þ≥£ ˝£¨aµƒœý∂‘‘≠◊”÷ ¡øŒ™M£¨‘Ú“ª∏ˆæß∞˚÷–a‘≠◊”µƒ ˝ƒøŒ™________£¨∏√æßõƒ√Ð∂»Œ™________(”√◊÷ƒ∏±Ì æ)°£
(1) 1s22s22p63s23p63d54s1 (2)¶“º¸ªÚº´–‘π≤º€º¸ SP3‘”ªØ (3)4 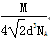
°æΩ‚Œˆ°ø
‘Â∑÷Œˆ£∫
(1)∏˘æð∫ÈÃÿπÊ‘ÚµƒÃÿ¿˝£¨dπϵ¿∞Î≥‰¬˙ ±∏¸Œ»∂®£¨∆‰µÁ◊”≈≈≤º Ω «1s22s22p63s23p63d54s1
∂¯≤ª «1s22s22p63s23p63d44s2°£
(2)SiO2÷–πË—ı÷ƺ‰µƒπ≤º€º¸ «°∞Õ∑∂‘Õ∑°±µƒ∑Ω Ω£¨À˘“‘ «¶“º¸£¨∆‰÷–Siµƒ‘”ªØ∑Ω Ω «SP3‘”ªØ°£
(3)”…∑÷ÃØ∑®º∆À„£∫8°¡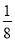 £´6°¡
£´6°¡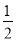 £Ω4£ª∏√æß∞˚µƒ÷ ¡øm£Ω
£Ω4£ª∏√æß∞˚µƒ÷ ¡øm£Ω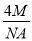 £¨∏√æß∞˚µƒ±þ≥§Œ™£∫2
£¨∏√æß∞˚µƒ±þ≥§Œ™£∫2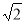 d,ê˝V£Ω(2
d,ê˝V£Ω(2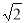 d)3£Ω16
d)3£Ω16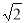 d3£¨æß∞˚√Ð∂»¶—£Ωm/V£Ω
d3£¨æß∞˚√Ð∂»¶—£Ωm/V£Ω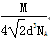
øºµ„£∫‘™Àÿ÷Ð∆⁄±Ì£¨æßõƒΩ·ππ
µ„∆¿£∫Âøº≤È‘™Àÿ÷Ð∆⁄±ÌµƒΩ·ππ£¨æßõƒΩ·ππ£¨æß∞˚µƒœýπÿº∆À„£¨ƒ—∂»÷–µ»°£±æµƒƒ—µ„‘⁄µ⁄£®3£©Œ £¨“™≥‰∑÷¿˚”√ƒøÀ˘∏¯Õº æ£¨Ω·∫œº∏∫ŒÕº–Œ“‘º∞ŒÔ¿Ìπ´ Ωµƒœýπÿº∆À„£¨≤≈ƒÐ’˝»∑Ω‚¥°£


 ƒÐøº ‘∆⁄ƒ©≥Â¥ÃæÌœµ¡–¥∞∏
ƒÐøº ‘∆⁄ƒ©≥Â¥ÃæÌœµ¡–¥∞∏
| ƒÍº∂ | ∏þ÷–øŒ≥à | ƒÍº∂ | ≥ı÷–øŒ≥à |
| ∏þ“ª | ∏þ“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı“ª | ≥ı“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ∂˛ | ∏þ∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı∂˛ | ≥ı∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ»˝ | ∏þ»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı»˝ | ≥ı»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
怺◊ª˘±˚œ©À·ı•œÀŒ¨æþ”–÷ «·°¢∆µ¬ ø̵»Ãÿ–‘£¨π„∑∫”¶”√”⁄÷∆◊˜π‚µºœÀŒ¨°£“—÷™AŒ™ƒ≥÷÷怺◊ª˘±˚œ©À·ı•œÀŒ¨µƒµ•Ã£¨∆‰◊™ªØπÿœµ»Áœ¬£∫
£®1£©AµƒΩ·ππºÚ ΩŒ™°°°°°°°°°°°°°° °£
£®2£©œ¬¡–”–πÿEµƒÀµ∑®≤ª’˝»∑µƒ «°°°°°°°°°°°°°°°°°°°°£®ÃÓ–¥◊÷ƒ∏±ý∫≈£©
A£ÆƒÐ πÀ·–‘∏þ√ÃÀ·ºÿ»Ð“∫Õ …´°°°°°°°°°° B£ÆƒÐ π‰ÂµƒCCl4»Ð“∫Õ …´
C£Æ“ª∂®Ãıº˛œ¬£¨ƒÐπª∑¢…˙œ˚»•∑¥”¶°°°°°° D£Æ“ª∂®Ãıº˛œ¬£¨ƒÐπª∑¢…˙»°¥˙∑¥”¶
£®3£©FµƒÕ¨∑÷“ÏππÃÂ÷– Ù”⁄ı•¿ýµƒ”–°°°°°°°°°°°°÷÷
£®4£©∑¥”¶¢⁄µƒªØ—ß∑Ω≥Ã Ω «°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°
∑¥”¶¢ðµƒªØ—ß∑Ω≥Ã Ω «°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2014-2015…Ω∂´ °◊Õ≤© –∏þ∂˛12‘¬‘¬øºªØ—ß ‘æÌ£®Ω‚Œˆ∞Ê£© –գ∫—°‘ÒÂ
‘⁄“ª∂®Œ¬∂»œ¬£¨“ª∂®¡øµƒÀÆ÷–£¨ ت“»È–¸◊«“∫¥Ê‘⁄œ¬¡–∆Ω∫‚£∫
Ca(OH)2(s) 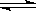 Ca2+(aq) + 20H-(aq)£¨µ±œÚ¥À–¸◊«“∫÷–º”»Î…Ÿ¡ø—ıªØ∏∆πÃà±£¨œ¬¡–Àµ∑®’˝»∑µƒ «
Ca2+(aq) + 20H-(aq)£¨µ±œÚ¥À–¸◊«“∫÷–º”»Î…Ÿ¡ø—ıªØ∏∆πÃà±£¨œ¬¡–Àµ∑®’˝»∑µƒ «
A£Æc£®Ca2+£©‘ˆ¥Û B£Æc£®Ca2+£©≤ª±‰
C£Æc£®OH-£©‘ˆ¥Û D£ÆCa(OH)2πÃõƒ÷ ¡ø≤ª±‰
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2014-2015…Ω∂´ °∂´”™ –∏þ“ª…œ—ß∆⁄µ⁄»˝¥Œƒ£øÈøº ‘ªØ—ß ‘æÌ£®Ω‚Œˆ∞Ê£© –գ∫—°‘ÒÂ
µ—È “ø…”√»ÁÕºÀ˘ 浃◊∞÷√∏…‘Ô°¢¥¢¥Ê∆¯ÃÂR£¨∂ý”ýµƒ∆¯ÃÂø…”√ÀÆŒ¸ ’£¨‘ÚR «

A£ÆNO2 B£ÆNO C£ÆSO2 D£ÆNH3
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2014-2015…Ω∂´ °∂´”™ –∏þ“ª…œ—ß∆⁄µ⁄»˝¥Œƒ£øÈøº ‘ªØ—ß ‘æÌ£®Ω‚Œˆ∞Ê£© –գ∫—°‘ÒÂ
œ¬¡–ŒÔ÷ ÷–£¨≤ªƒÐ”…µ•÷ ÷±Ω”ªØ∫œ…˙≥…µƒ «
¢ŸCuS ¢⁄FeS ¢€SO3 ¢ÐH2S ¢ðFeCl2
A°¢¢Ÿ¢€¢ð B°¢¢Ÿ¢⁄¢€¢ð C°¢¢Ÿ¢⁄¢Ð¢ð D°¢»´≤ø
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2014-2015∫”±± °–œÃ® –∏þ∂˛12‘¬‘¬øºªØ—ß ‘æÌ£®Ω‚Œˆ∞Ê£© –գ∫—°‘ÒÂ
’˝≈À·(H3BO3) «“ª÷÷∆¨≤„◊¥Ω·ππ∞◊…´æßã¨≤„ƒ⁄µƒH3BO3∑÷◊”Õ®π˝«‚º¸œý¡¨(»Áœ¬Õº)°£œ¬¡–”–πÿÀµ∑®’˝»∑µƒ «
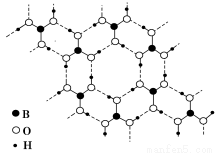
A£Æ’˝≈À·æßàٔ⁄‘≠◊”æßÃÂ
B£ÆH3BO3∑÷◊”µƒŒ»∂®–‘”Ϋ‚º¸”–πÿ
C£Æ∑÷◊”÷–≈‘≠◊”◊ÓÕ‚≤„Œ™8µÁ◊”Œ»∂®Ω·ππ
D£Æ∫¨1 mol H3BO3µƒæßÃÂ÷–”–3 mol«‚º¸
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2014-2015∫”±± °–œÃ® –∏þ∂˛12‘¬‘¬øºªØ—ß ‘æÌ£®Ω‚Œˆ∞Ê£© –գ∫—°‘ÒÂ
¿Î◊”æßÃÂŒ»∂®–‘»°æˆ”⁄æßÃÂ÷–æß∏҃еƒ¥Û–°°£≈–∂œKCl°¢NaCl°¢CaO°¢BaOÀƒ÷÷æßÃÂŒ»∂®–‘”…∏þµΩµÕµƒÀ≥–Ú «
A£ÆKCl£æNaCl£æBaO£æCaO B£ÆNaCl£æKCl£æCaO£æBaO
C£ÆCaO£æBaO£æNaCl£æKCl D£ÆCaO£æBaO£æKCl£æNaCl
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2014-2015…œ∫£ –„…––«¯∏þ∂˛µ⁄“ª—ß∆⁄∆⁄ƒ©øº ‘ªØ—ß ‘æÌ£®Ω‚Œˆ∞Ê£© –գ∫º∆À„Â
Al2O3µƒ÷ ¡ø∑÷ ˝ £®”√–° ˝±Ì 棨–° ˝µ„∫Û±£¡Ù¡ΩŒª£©°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫2014-2015…œ∫£ –„…––«¯∏þ∂˛µ⁄“ª—ß∆⁄∆⁄ƒ©øº ‘ªØ—ß ‘æÌ£®Ω‚Œˆ∞Ê£© –գ∫—°‘ÒÂ
Ω´¬»ªØ¬¡»Ð“∫∫Õ¡ÚÀ·¬¡»Ð“∫∑÷±’Ù∏…°¢◊∆…’£¨◊Ó∫Ûµ√µΩµƒπÃà«
A£Æ∂º «Al2O3 B£Æ“¿¥ŒŒ™AlCl3°¢Al2(SO4)3
C£Æ∂º «Al(OH)3 D£Æ“¿¥ŒŒ™Al2O3°¢Al2(SO4)3
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
∞Ÿ∂»÷¬–≈ - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒÞ÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com