
ij�о���ѧϰС�������Fe(OH)3������Ʊ���
���������Ȼ�����Һ�����ˮ��ʱ��Һ���Ϊ________ɫ���õ�����_______ _����Ӧ�����ӷ���ʽΪ________________________________________________________���ô˷�ɢϵ����ʵ�飺
_����Ӧ�����ӷ���ʽΪ________________________________________________________���ô˷�ɢϵ����ʵ�飺
(1)����װ��U�ι��ڣ���ʯī���缫����ֱͨ����Դ��ͨ��һ��ʱ���������������ɫ________________________________________________________________________��
�����_____________________________________________________________��
���������Ϊ___________________________________________________________��
(2)�����м��뱥�� ���������Һ��������������__________________________
���������Һ��������������__________________________
________________________________________________________________________��
ԭ����____________________________________________________________��
(3)��������μ������ϡ���ᣬ������___________________________________
________________________________________________________________________��
ԭ����___________________________________________________________
____________________________ ____________________________________________��
____________________________________________��
(4)�ᴿ�˷�ɢϵ�ķ�����___________________________________________________��
��ѡ�ᴿװ���������е�________��

������ʵ�����Ʊ�Fe(OH)3����IJ��� Ҫ�㣺����ˮ����У��ټ��뱥�͵�FeCl3��Һ��������������ɫ��ֹͣ���ȡ�
Ҫ�㣺����ˮ����У��ټ��뱥�͵�FeCl3��Һ��������������ɫ��ֹͣ���ȡ�
(1)Fe(OH)3�������Ӵ�����ɣ��ڵ糡�����·�����Ӿ��������ɵĽ����������ƶ���������������ɫ���
(2)�������Һ�д������������ӣ�������������ɣ�Fe(OH)3������������ɣ���������кͣ�Fe(OH)3���巢���۳������γ�Fe(OH)3������
(3)�����д���H����SO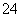 ��һ����SO
��һ����SO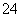 �ĸ�����к�Fe(OH)3��������������ɣ�����۳�����һ����Fe(OH)3��һ�ּ����H����Ӧ���ʳ����ܽ⡣
�ĸ�����к�Fe(OH)3��������������ɣ�����۳�����һ����Fe(OH)3��һ�ּ����H����Ӧ���ʳ����ܽ⡣
(4)���ڽ�������ֱ����С������Һ��ɢ�ʺ���Һ��ɢ��֮�䣬���ᴿ����ͨ��������������
�𰸣���֡�Fe(OH)3����
Fe3����3H2O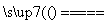 Fe(OH)3(����)��3H��
Fe(OH)3(����)��3H��
(1)���Fe(OH)3����������ɡ���Ӿ
(2)�γɺ��ɫ����������ʵ�����������к��˽�������������ɣ�ʹFe(OH)3����۳�
(3)�ȳ��ֺ��ɫ������������ܽ⣬���ػ�ɫ��Һ�������ʹFe(OH)3����۳�������ϡ����ļ��룬H����Fe(OH)3������Ӧ��Fe(OH)3��3H��===Fe3����3H2O��ʹ�����ܽ�
(4)������E



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�����п��÷�Һ©�����룬���л���Ӧ�ӷ�Һ©�����Ͽڵ�������(����)
A��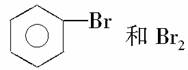
B��CH3CH2CH2CH2Cl��H2O
C��CCl4��CHCl3
D��CH2Br��CH2Br��NaBr(H2O)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȥþ���е��������ۣ���ѡ��(����)
A������
B����ˮ
C������
D������������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2O��NaOH��Na2CO3��NaCl��Na2SO4�ɰ�ij�ֱ���Ϊͬһ�����ʣ����з������ȷ����(����)
���ƵĻ�������������ᷴӦ�����ʡ��ۿ�����ˮ�����ʡ��ܵ���ʡ������Ρ����Ƶĺ���������
A���٢ۢܢ� B���٢ڢݢ�
C���ڢݢ� D���٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڽ������������ȷ����(����)
A�����߲��������壬���ܷ������������
B���������ķ�����������ʱ��ʹ�õİ�Ĥֻ���ý�С�ķ��ӡ�����ͨ��
C�������˶��ǽ����������е��˶���ʽ�����Ծݴ˰ѽ������Һ������Һ������
D���������Ӿ��нϴ�ı�����������������ӻ������ӣ����ڵ糡�����»������Ӿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ָ����Ӧ�����ӷ���ʽ��ȷ����(����)
A��Cu����ϡHNO3��
Cu��2H����NO ===Cu2����NO2����H2O
===Cu2����NO2����H2O
B��(NH4)2Fe(SO4)2��Һ�����NaOH��Һ��Ӧ��
Fe(OH)2��Fe2����2OH��===Fe(OH)2��
C����CH3COOH�ܽ�CaCO3��
CaCO3��2H��===Ca2����H2O��CO2��
D����NaAlO2��Һ��ͨ�����CO2��Al(OH)3��
CO2��AlO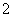 ��2H2O===Al(OH)3����HCO
��2H2O===Al(OH)3����HCO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ǧ���仯������������ء������豸��X���߷������ϵȡ��ش��������⣺
(1)Ǧ��̼��ͬ��Ԫ�أ���̼��4�����Ӳ㡣Ǧ��Ԫ�����ڱ���λ��Ϊ��________���ڡ���________�壻PbO2�����Ա�CO2������________(�ǿ��������)��
(2)PbO2��Ũ���Ṳ�����ɻ���ɫ���壬��Ӧ�Ļ�ѧ����ʽΪ_______________________��
(3)PbO2����PbO�����������Һ��Ӧ�Ƶã���Ӧ�����ӷ���ʽΪ___________________��PbO2Ҳ����ͨ��ʯīΪ�缫��Pb(NO3)2��Cu(NO3)2�Ļ����ҺΪ���Һ�����ȡ�����������ĵ缫��ӦʽΪ____________________�������Ϲ۲쵽��������____________________�������Һ�в�����Cu(NO3)2�����������ĵ缫��ӦʽΪ______________________________������������Ҫȱ����____________________��
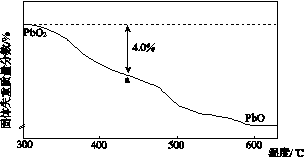
(4)PbO2�ڼ��ȹ��̷����ֽ��ʧ����������ͼ��ʾ����֪ʧ�������ϵ�a��Ϊ��Ʒʧ��4.0%(��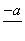 ��100%)�IJ������塣��a�������ɱ�ʾΪPbOx��mPbO2��nPbO����ʽ����xֵ��m��nֵ_______________________________________
��100%)�IJ������塣��a�������ɱ�ʾΪPbOx��mPbO2��nPbO����ʽ����xֵ��m��nֵ_______________________________________
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ת������Դ���úͻ�����������Ҫ�о����⡣�������������ж��ַ�����
(1)���ռ�����H2S�����Һ���뵽��ͼ��ʾ�ĵ��ص����������е�⡣���������������������·�Ӧ��
S2����2e��===S��(n��1)S��S2��===S

��д�����ʱ�����ĵ缫��Ӧʽ��________________��
�ڵ�������������Һ��ϡ�����ữ�õ����ʣ������ӷ���ʽ��д��__________________________��
(2)��H2S�Ϳ����Ļ������ͨ��FeCl3��FeCl2��CuCl2�Ļ����Һ�з�Ӧ����S��������ת����ͼ��ʾ��
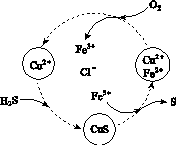
����ͼʾ��ת���У����ϼ۲����Ԫ����________��
�ڷ�Ӧ�е���1 mol H2Sת��Ϊ����ʱ��������Һ��Fe3�������ʵ������䣬������O2�����ʵ���Ϊ________��
�����¶�һ���Ͳ�������Һ�������£�����ͨ�������壬����ֽ��衣��ʹ���ɵ������в���CuS���ɲ�ȡ�Ĵ�ʩ��________________��
(3)H2S�ڸ����·ֽ�������������H2������Ӧ�ڲ�ͬ�¶��´ﵽƽ��ʱ����������и���ֵ����������ͼ��ʾ��H2S�ڸ����·ֽⷴӦ�Ļ�ѧ����ʽΪ__________________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȷ��ʾ���з�Ӧ�����ӷ���ʽΪ
A������������ϡ�����У�FeS+2H+=Fe2++H2S��
B���Ȼ�����Һ�м��������ˮ��Al3++4NH3��H2O=AlO2-+4NH4++2H2O
C�������������Һ�е���ϡ���S2O32-+2H+=S��+SO2��+H2O
D����NaHSO4��Һ�еμ�Ba(OH)2�����ԣ�H++SO42-+Ba2++OH-=BaSO4��+H2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com