
»ÆѧŹĒŅ»ĆÅŅŌŹµŃéĪŖÖ÷µÄæĘѧ£¬»ÆѧŹµŃéŹĒѧĻ°Ģ½¾æĪļÖŹŠŌÖŹµÄ»ł±¾·½·ØÖ®Ņ»”£
£Ø1£©ĻĀĮŠÓŠ¹ŲŠšŹöÕżČ·µÄŹĒ__________£ØĢīŠ“ŠņŗÅ£©
a£®Ź¹ÓĆĶŠÅĢĢģĘ½µÄµŚŅ»²½²Ł×÷ŹĒ½«ÓĪĀėŅĘÖĮ±ź³ßĮćæĢ¶Č“¦
b£®¹żĀĖ²Ł×÷¹ż³ĢÖŠ£¬ĪŖ¼Óæģ¹żĀĖĖŁ¶ČæÉÓĆ²£Į§°ō¶ŌĀ©¶·ÖŠµÄČÜŅŗ½ųŠŠ½Į°č
c£®ÓĆÅØĮņĖįÅäÖĘĻ”ČÜŅŗŹ±£¬ŌŚĮæĶ²ÖŠŗāĻ”ŗóŅŖĄäČ“ÖĮŹŅĪĀŌŁ×ŖŅʵ½ČŻĮæĘæÖŠ
d£®ÓĆČŻĮæĘæÅäÖĘČÜŅŗŹ±£¬¶ØČŻŗóŅ”ŌČŅŗĆęĻĀ½µ£¬ŌŁ¼ÓÕōĮóĖ®ÖĮæĢ¶ČĻß“¦£¬ĖłµĆČÜŅŗÅضČĘ«µĶ
£Ø2£©ĻÖÓŠĮłÖÖĘųĢå£ŗH2”¢O2”¢NH3”¢SO2”¢NO2”¢NO”£æÉŅŌĄūÓĆČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹÕ¼Æ”£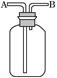
¢ŁČōĘųĢå“ÓBæŚ½ųČė£¬æÉŹÕ¼ÆµÄĘųĢåŹĒ_______________£»
¢ŚČōŌŚÉÕĘæ֊עĀśĖ®£¬ŌņĘųĢåÓ¦øĆ“Ó______£ØĢīŠ“”°A”±»ņ”°B”±£©æŚ½ųČė£¬æÉŅŌŹÕ¼ÆµÄĘųĢåŹĒ_________________________”£
 ¼ā×ÓÉśŠĀæĪĢĆæĪŹ±×÷ŅµĻµĮŠ“š°ø
¼ā×ÓÉśŠĀæĪĢĆæĪŹ±×÷ŅµĻµĮŠ“š°ø Ó¢²Å¼Ę»®Ķ¬²½æĪŹ±øߊ§ŃµĮ·ĻµĮŠ“š°ø
Ó¢²Å¼Ę»®Ķ¬²½æĪŹ±øߊ§ŃµĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠ¹ŲÓŚŹµŃéŌĄķ»ņ²Ł×÷µÄŠšŹöÖŠ£¬²»ÕżČ·µÄŹĒ
| A£®ÅŠ¶ĻŌķ»Æ·“Ó¦ŹĒ·ńĶźČ«£¬æÉČ”·“Ó¦ŗóµÄ»ģŗĻŅŗµĪČėČČĖ®ÖŠ |
| B£®¼õŃ¹¹żĀĖ²»ŅĖ¹żĀĖ½ŗד³Įµķ»ņæÅĮ£Ģ«Š”µÄ³Įµķ |
| C£®ÖʱøĮņĖįŃĒĢśļ§¾§Ģ壬Šč¼ÓČČÖĮ“óĮ澧ĢåĪö³ö²¢Ź£ÓąÉŁĮæŅŗĢ弓Ķ£Ö¹¼ÓČČ |
| D£®Ņ×Č¼ŹŌ¼ĮÓėĒæŃõ»ÆŠŌŹŌ¼ĮŠč·ÖæŖ·ÅÖĆ£¬²¢Ō¶Ąė»šŌ“£»½šŹō×Å»šŹ±.æÉÓĆĻøɳø²øĒĆš»š |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijĢ½¾æŠ”×éŌŚŹµŃéŹŅÖŠÓĆĀĮĶĮæó(Ö÷ŅŖ³É·ÖĪŖAl2O3£¬»¹ŗ¬ÓŠFe2O3”¢SiO2)ĢįČ”Ńõ»ÆĀĮ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŌŚŹµŃéÖŠŠčÓĆ1 mol”¤L£1µÄNaOHČÜŅŗ480 mL£¬ÅäÖĘøĆČÜŅŗŅŃÓŠĻĀĮŠŅĒĘ÷£ŗĶŠÅĢĢģĘ½(ķĄĀė)”¢½ŗĶ·µĪ¹Ü”¢Ņ©³×”¢²£Į§°ō£¬»¹Č±ÉŁµÄŅĒĘ÷ŹĒ ”£
ŌŚ×ĘÉÕ²Ł×÷ÖŠÓƵ½ĻĀĮŠŅĒĘ÷ÖŠµÄŅ»ÖÖ£¬ĘäĆū³ĘŹĒ ”£
(2)Š“³ö²½Öč¢ŁÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
(3)²Ł×÷¢ŪÖŠµÄĻ“µÓ²Ł×÷ČēŗĪ½ųŠŠ£æ ”£
(4)¼×Ķ¬Ń§ŌŚŹµŃéŹŅÖŠÓĆČēĶ¼×°ÖĆÖʱøCO2ĘųĢ壬²¢ĶØČėĀĖŅŗBÖŠÖʱøAl(OH)3Ź±£¬½į¹ūƻӊ²śÉśŌ¤ĘŚĻÖĻó”£
ŅŅĶ¬Ń§·ÖĪöČĻĪŖ£ŗ¼×Ķ¬Ń§ĶØČėCO2µÄĮæ²»×ćŹĒµ¼ÖĀŹµŃ鏧°ÜµÄŌŅņÖ®Ņ»£¬ÄćČĻĪŖŅŅµÄ·ÖĪöŹĒ·ńŗĻĄķ£æ ”£ČōŗĻĄķ£¬ĒėÓĆĄė×Ó·½³ĢŹ½½āŹĶĘäŌŅņ (ČōÄćČĻĪŖ²»ŗĻĄķ£¬øĆæÕ²»×÷“š)”£
±ūĶ¬Ń§·ÖĪöČĻĪŖ£ŗ¼×Ķ¬Ń§ĶØČėµÄCO2ÖŠŗ¬ÓŠHClĘųĢ壬Ņ²ŹĒµ¼ÖĀŹµŃ鏧°ÜµÄŌŅņ£¬ŌŚŹµŃéÖŠŌö¼ÓijװÖĆæɽā¾öÕāøöĪŹĢā”£Ēė°ļÖś±ūĶ¬Ń§»³öøĆ×°ÖĆĶ¼£¬²¢×¢Ć÷ŹŌ¼ĮĆū³Ę”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéŹŅÓūÅäÖĘ100 mL”¢1mol£ÆLµÄNaClČÜŅŗ£¬Ēė»Ų“š£ŗ
£Ø1£©²»ŠčŅŖŹ¹ÓƵÄŅĒĘ÷ŹĒ ”£
A”¢ÉÕ±B”¢500 mLČŻĮæĘæC”¢ĮæĶ² D”¢½ŗĶ·µĪ¹Ü E”¢²£Į§°ō F”¢100 mLČŻĮæĘæ
£Ø2£©ÅäÖĘŹ±ÓĆĶŠÅĢĢģĘ½Ó¦³ĘČ”NaCl g”£
£Ø3£©ÅäÖĘČÜŅŗµÄ²Ł×÷Ė³ŠņŹĒ(ÓĆ×ÖÄø±ķŹ¾) ”£
A”¢³ĘĮæ B”¢Ļ“µÓ C”¢¶ØČŻ D”¢Čܽā E”¢Ņ”ŌČ F”¢×ŖŅĘ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
×ī½ü£¬ĪŅ¹śĄūÓĆÉś²śĮ×ļ§ÅŷŵķĻŌüĮ׏ÆøąÖĘČ”ĮņĖį²¢ĮŖ²śĖ®ÄąµÄ¼¼ŹõŃŠ¾æ»ńµĆ³É¹¦”£¾ßĢåÉś²śĮ÷³ĢČēĶ¼£ŗ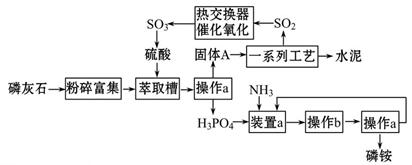
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©²Ł×÷aµÄĆū³ĘŹĒ___________£¬ŹµŃéŹŅÖŠ½ųŠŠ“Ė²Ł×÷µÄ·Ē²£Į§ŅĒĘ÷»ņÓĆĘ·ÓŠ________________£»ŌŚŹµŃéŹŅÖŠ²Ł×÷bµÄĆū³ĘŹĒ______________________”£
£Ø2£©×°ÖĆaÖŠÉś³ÉĮ½ÖÖĖįŹ½ŃĪ£¬ĖüĆĒµÄ»ÆѧŹ½·Ö±šŹĒ_______________”£
£Ø3£©ŅĄĢāŅā²Ā²ā¹ĢĢåAÖŠŅ»¶Øŗ¬ÓŠµÄĪļÖŹµÄ»ÆѧŹ½ŹĒ____________________(½į¾§Ė®²æ·Ö²»Š“)”£
£Ø4£©ČČ½»»»Ę÷ŹĒŹµĻÖĄäČČ½»»»µÄ×°ÖĆ”£»ÆѧŹµŃéÖŠŅ²¾³£ĄūÓĆČČ½»»»Ą“ŹµĻÖijÖÖŹµŃéÄæµÄ£¬Ęų”¢ŅŗČČ½»»»Ź±Ķس£Ź¹ÓƵÄŅĒĘ÷ŹĒ________________________”£
£Ø5£©ÖĘĮņĖįĖł²śÉśµÄĪ²Ęų³żĮĖŗ¬ÓŠN2”¢O2Ķā£¬»¹ŗ¬ÓŠSO2£¬Ī¢ĮæµÄSO3ŗĶĖįĪķ”£ÄÜÓĆÓŚ²ā¶ØĮņĖįĪ²ĘųÖŠSO2ŗ¬ĮæµÄŹĒ___________________”£
| A£®NaOHČÜŅŗ”¢·ÓĢŖŹŌŅŗ | B£®KMnO4ČÜŅŗ”¢Ļ”ĮņĖį |
| C£®µāĖ®”¢µķ·ŪČÜŅŗ | D£®°±Ė®”¢·ÓĢŖŹŌŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
×÷ĪŖøßÖŠÉś£¬Ń§»įĄūÓĆĪŅĆĒæĪĢĆÉĻѧµ½µÄÖŖŹ¶Ą“½ā¾öÉś»īÖŠµÄŅ»Š©ĪŹĢā£¬ŹĒĪŅĆĒѧĻ°µÄÖŲŅŖÄæµÄÖ®Ņ»”£ÖŲĒģŅ»ÖŠÄ³»ÆѧŹµŃéŠĖȤŠ”×飬Ņ»ŠŠĖÄČĖ£¬ĄūÓĆŹµŃéŹŅĄĻŹ¦Ģį¹©µÄ»ł±¾ŅĒĘ÷ŗĶŅ©Ę·£¬×ŌŠŠ¹ŗÖĆĮĖ¼¦µ°£¬Ź³“×µČÉś»īÓĆĘ·£¬½ųŠŠĮĖČēĻĀĢ½¾æ”£
I. ¼×Ķ¬Ń§ĄĻ¼ŅŌŚÉ½Ī÷£¬¶Ō¶łŹ±ŌŚ¼ŅĻēĘ·³¢µ½µÄɽĪ÷ĄĻ³Ā“×µÄ×ĢĪ¶¼ĒŅäÓĢŠĀ£¬øśĖęøøÄøĄ“µ½ÖŲĒģŗó£¬×ÜŹĒ¾õµĆ³¬ŹŠĀņµ½µÄ“ײ»Čē¶łŹ±µÄĪ¶µĄ£¬²éŌÄĻą¹Ų׏ĮĻŗ󣬵ĆÖŖŅŌĻĀŠÅĻ¢£ŗ
¢Ł“×·ÖĮ½ÖÖ£¬ÄšŌģ“×ŗĶÅäÖʓה£Õż×ŚµÄĄĻ³Ā“×±ŲŠė¾³¤¾ĆŹ±¼äÄšŌģ²ÅµĆ“ĖĆĄĪ¶£¬ŹŠ³”ÉĻ¶ą³ä³ā׏¤Ņµ“×Ėį¼ÓĖ®¹“¶ŅµÄÅäÖʓה£
¢ŚÄšŌģ“×¹ś¼Ņ±ź×¼ĪŖ“×Ėįŗ¬Įæ±ŲŠė“óÓŚ3.50 g/100mL£¬¶ųÅäÖĘ“×¹ś¼Ņ±ź×¼½öĪŖ1.50 g”«3.50g/100mL”£
¢ŪŌŚĄĻŹ¦µÄ°ļÖśĻĀ£¬²ā¶ØĮĖ³¬ŹŠ¹ŗĀņµÄŹ³“×ÖŠ£¬“×ĖįµÄĪļÖŹµÄĮæÅضČĪŖ0.75mol/L”£
£Ø1£©Ēė°ļÖśÕÅĶ¬Ń§¼ĘĖć“Ó³¬ŹŠ¹ŗĀņµÄŹ³“×ÖŠ“×Ėįŗ¬ĮæĪŖ_______g/100mL£¬ŹōÓŚ____________“×£ØĢī”°ÄšŌģ”±»ņ”°ÅäÖĘ”±£©”££ØĢįŹ¾£ŗ“×ĖįĦ¶ūÖŹĮæĪŖ60g/mol£©
£Ø2£©ĒėŠ“³ö“×ĖįÓė¼¦µ°æĒ(Ö÷ŅŖ³É·ÖĪŖCaCO3)·“Ó¦µÄĄė×Ó·½³ĢŹ½_______________________________”£
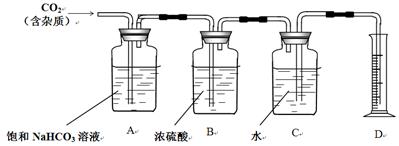
II. ĻĀĶ¼ŹĒÖŲĒģŅ»ÖŠ»ÆѧŹµŃéŹŅÅØŃĪĖįŹŌ¼Į±źĒ©ÉĻµÄ²æ·ÖÄŚČŻ”£ŅŅĶ¬Ń§ĻÖÓĆøĆÅØŃĪĖįÅä100mL1mol/LµÄĻ”ŃĪĖį”£æɹ©Ń”ÓƵÄŅĒĘ÷ÓŠ£ŗ¢Ł½ŗĶ·µĪ¹Ü£»¢ŚÉÕĘ棻¢ŪÉÕ±£»¢ÜŅ©³×£»¢ŻĮæĶ²£»¢ŽĶŠÅĢĢģĘ½£»¢ß²£Į§°ō”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÅäÖĘĻ”ŃĪĖįŹ±£¬»¹Č±ÉŁµÄŅĒĘ÷ÓŠ £»
£Ø2£©¾¼ĘĖć£¬ÅäÖĘ100mL1mol/LµÄĻ”ŃĪĖįŠčŅŖÓĆĮæĶ²ĮæČ”ÉĻŹöÅØŃĪĖįµÄĢå»żĪŖ mL(±£ĮōŠ”ŹżµćŗóŅ»Ī»)£»
£Ø3£©¶ŌĖłÅäÖʵÄĻ”ŃĪĖį½ųŠŠ²ā¶Ø£¬·¢ĻÖĘäÅØ¶ČŠ”ÓŚ1mol/L£¬ŅżĘšĪó²īµÄŌŅņæÉÄÜŹĒ ”£
| A£®¶ØČŻŹ±ø©ŹÓČŻĮæĘææĢ¶ČĻß |
| B£®ČŻĮæĘæŌŚŹ¹ÓĆĒ°Ī“øÉŌļ£¬ĄļĆęÓŠÉŁĮæÕōĮóĖ® |
| C£®×ŖŅĘČÜŅŗŗó,Ī“Ļ“µÓÉÕ±ŗĶ²£Į§°ō |
| D£®¶ØČŻŅ”ŌČŗó·¢ĻÖŅŗĆęµĶÓŚČŻĮæĘæµÄæĢ¶ČĻߣ¬ŌŁ¼ÓĖ®ÖĮæĢ¶ČĻß |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŃĒĀČĖįÄĘ£ØNaClO2£©ŹĒŅ»ÖÖÖŲŅŖµÄŗ¬ĀČĻū¶¾¼Į£¬ŌŚ¼īŠŌČÜŅŗÖŠNaClO2±Č½ĻĪČ¶Ø£¬Ö÷ŅŖÓĆÓŚĖ®µÄĻū¶¾ŅŌ¼°É°ĢĒ”¢ÓĶÖ¬µÄĘÆ°×Óėɱ¾ś”£ŅŌĻĀŹĒ¹żŃõ»ÆĒā·ØÉś²śŃĒĀČĖįÄĘµÄ¹¤ŅÕĮ÷³ĢĶ¼£ŗ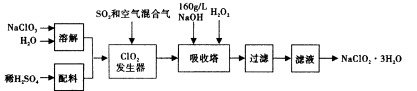
ŅŃÖŖ£ŗ¢ŁNaClO2µÄČܽā¶ČĖęĪĀ¶ČÉżø߶ųŌö“ó£¬ŹŹµ±Ģõ¼žĻĀæɽį¾§Īö³öNaClO2”¤3H2O”£
¢Ś“æClO2Ņ×·Ö½ā±¬ÕØ£¬Ņ»°ćÓĆĻ”ÓŠĘųĢå»ņæÕĘųĻ”ŹĶµ½10%ŅŌĻĀ°²Č«”£
¢Ū160 g/L NaOHČÜŅŗŹĒÖø160 g NaOH¹ĢĢåČÜÓŚĖ®ĖłµĆČÜŅŗµÄĢå»żĪŖ1L”£
£Ø1£©160 g/L NaOHČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ____”£ČōŅŖ¼ĘĖćøĆČÜŅŗµÄÖŹĮæ·ÖŹż£¬»¹ŠčŅŖµÄŅ»øöĢõ¼žŹĒ____£ØÓĆĪÄ×ÖĖµĆ÷£©”£
£Ø2£©·¢ÉśĘ÷ÖŠ¹ÄČėæÕĘųµÄ×÷ÓĆæÉÄÜŹĒ____£ØŃ”ĢīŠņŗÅ£©”£
a£®½«SO2Ńõ»Æ³ÉSO3£¬ŌöĒæĖįŠŌ£»
b£®Ļ”ŹĶClO2ŅŌ·ĄÖ¹±¬ÕØ£»
c£®½«NaClO3Ńõ»Æ³ÉClO2
£Ø3£©ĪüŹÕĖžÄŚµÄ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____”£ĪüŹÕĖžµÄĪĀ¶Č²»Äܳ¬¹ż20”ę£¬ĘäÄæµÄŹĒ ”£
£Ø4£©ĪüŹÕĖžÖŠÓ¦Ī¬³ÖNaOHÉŌ¹żĮ棬ĄķÓÉŹĒ____”£
£Ø5£©ĪüŹÕĖžÖŠÄÜ·ńÓĆFeCl2“śĢęH2O2____£ØĢīÄÜ£®·ń£©?ĄķÓÉŹĒ____”£
£Ø6£©“ÓĀĖŅŗÖŠµĆµ½NaClO2”¤3H2O“Ö¾§ĢåµÄŹµŃé²Ł×÷ŅĄ“ĪŹĒ____£ØŃ”ĢīŠņŗÅ£©”£
A£®ÕōĮó b£®Õō·¢ c£®×ĘÉÕ d£®¹żĀĖ e£®ĄäČ“½į¾§
ŅŖµĆµ½øü“æµÄNaClO2”¤3H2O¾§Ģå±ŲŠė½ųŠŠµÄ²Ł×÷ŹĒ____£ØĢī²Ł×÷Ćū³Ę£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©Įņ“śĮņĖįÄĘ£ØNa2S2O3”¤5H2O£©Ė×Ćū”°“óĖÕ“ņ”±£¬ÓÖ³ĘĪŖ”°ŗ£²Ø”±”£ĖüŅ×ČÜÓŚĖ®£¬ÄŃČÜÓŚŅŅ“¼£¬¼ÓČČŅ×·Ö½ā”£¹¤ŅµÉĻ³£ÓĆŃĒĮņĖįÄĘ·Ø”¢Įņ»Æ¼ī·ØµČÖʱø”£Ä³ŹµŃéŹŅÄ£Äā¹¤ŅµĮņ»Æ¼ī·ØÖĘČ”Įņ“śĮņĖįÄĘ£¬Ę䷓ӦװÖĆ¼°ĖłŠčŹŌ¼ĮČēĻĀĶ¼£ŗŹµŃé¾ßĢå²Ł×÷²½ÖčĪŖ£ŗ
¢ŁæŖĘō·ÖŅŗĀ©¶·£¬Ź¹ĮņĖįĀżĀżµĪĻĀ£¬ŹŹµ±µ÷½ŚĀŻŠż¼Š£¬Ź¹·“Ó¦²śÉśµÄSO2ĘųĢå½Ļ¾łŌȵŲĶØČėNa2SŗĶNa2CO3µÄ»ģŗĻČÜŅŗÖŠ£¬Ķ¬Ź±æŖĘōµē“ŽĮ°čĘ÷½Į¶Æ”£
¢ŚÖĮĪö³öµÄĮņ²»ŌŁĻūŹ§£¬æŲÖĘČÜŅŗµÄpH½Ó½ü
7Ź±£¬Ķ£Ö¹ĶØČėSO2ĘųĢ唣
¢Ū³éĀĖĖłµĆµÄĀĖŅŗ£¬×ŖŅĘÖĮÕō·¢ĆóÖŠ£¬Ė®Ō”¼ÓČČ
ÅØĖõ£¬Ö±µ½ČÜŅŗ±ķĆę³öĻÖ¾§Ä¤”£
¢ÜĄäČ“½į¾§”¢³éĀĖ”¢Ļ“µÓ”£
¢Ż½«¾§Ģå·ÅČėŗęĻäÖŠ£¬ŌŚ40”«45”ę×óÓŅøÉŌļ
50”«60min£¬³ĘĮ攣
Ēė»Ų“šŅŌĻĀĪŹĢā£ŗ
£Øl£©ŅĒĘ÷aµÄĆū³ĘŹĒ £»
£Ø2£©²½Öč¢ŚÖŠČōæŲÖĘpHÖµŠ”ÓŚ7£¬Ōņ²śĀŹ»įĻĀ½µ£¬ĒėÓĆĄė×Ó·½³ĢŹ½½āŹĶŌŅņ£ŗ ”£
£Ø3£©²½Öč¢ŪÖŠ²»Äܽ«ČÜŅŗÕō·¢ÖĮøɵÄŌŅņŹĒ £»¾§Ä¤Ķس£ŌŚČÜŅŗ±ķĆę³öĻÖµÄŌŅņŹĒ ”£
£Ø4£©²½Öč¢ÜÖŠĻ“µÓĮņ“śĮņĖįÄĘ¾§ĢåĖłÓĆŹŌ¼ĮµÄ½į¹¹Ź½ŹĒ ”£
£Ø5£©ĪŖ¼ģŃéÖĘµĆµÄ²śĘ·µÄ“æ¶Č£¬øĆŹµŃ銔×é³ĘČ”5,0æĖµÄ²śĘ·ÅäÖĘ³É250mLĮņ“śĮņĖįÄĘČÜŅŗ£¬²¢ÓĆ¼ä½ÓµāĮæ·Ø±ź¶ØøĆČÜŅŗµÄÅØ¶Č£ŗŌŚ×¶ŠĪĘæÖŠ¼ÓČė25mL 0£®0lmol”¤L-1 KIO3ČÜŅŗ£¬²¢¼ÓČė¹żĮæµÄKI²¢Ėį»Æ£¬·¢ÉśĻĀĮŠ·“Ó¦£ŗ5I£+IO3£+6H+=3I2+3H2O£¬ŌŁ¼ÓČė¼øµĪµķ·ŪČÜŅŗ£¬Į¢¼“ÓĆĖłÅäNa2S2O3ČÜŅŗµĪ¶Ø£¬·¢Éś·“Ó¦£ŗI2+2S2O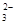 =2I£+S4O
=2I£+S4O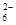 £¬µ±Ą¶É«ĶŹČ„H°ė·ÖÖÓ²»±äÉ«Ź±µ½“ļµĪ¶ØÖÕµć”£ŹµŃ鏿¾ŻČēĻĀ±ķ£ŗ
£¬µ±Ą¶É«ĶŹČ„H°ė·ÖÖÓ²»±äÉ«Ź±µ½“ļµĪ¶ØÖÕµć”£ŹµŃ鏿¾ŻČēĻĀ±ķ£ŗ
| ŹµŃéŠņŗÅ | 1 | 2 | 3 |
| Na2S2O3ČÜŅŗĢå»ż£ØmL£© | 19.98 | 20.02 | 21.18 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ŹµŃéŹŅŠčŅŖ0£®2mol/L CuSO4ČÜŅŗ250ml£¬ŹµŃéŹŅæÉĢį¹©ÅäÖĘČÜŅŗµÄŹŌ¼ĮÓŠ£ŗ¢ŁĄ¶É«µØ·Æ¾§Ģå(CuSO4”¤5H2O) ¢Ś4mol/L CuSO4ČÜŅŗ
(1)ĪŽĀŪ²ÉÓĆŗĪÖÖŹŌ¼Į½ųŠŠÅäÖĘ£¬ŹµŃé±ŲŠėÓƵ½µÄ²£Į§ŅĒĘ÷³żÉÕ±”¢²£Į§°ō”¢½ŗĶ·µĪ¹ÜĶā£¬ÖĮÉŁ»¹ŠčŅŖµÄŅ»ÖÖŅĒĘ÷ŹĒ________£¬ŌŚŹ¹ÓĆøĆŅĒĘ÷Ē°±ŲŠė½ųŠŠµÄ²Ł×÷ŹĒ ”£
(2)ČōÓĆµØ·Æ¾§Ģå½ųŠŠÅäÖĘ£¬ŠčÓĆĶŠÅĢĢģĘ½³ĘČ”CuSO4”¤ 5H2OµÄÖŹĮæĪŖ________g£»Čē¹ūÓĆ4mol/LµÄCuSO4ČÜŅŗĻ”ŹĶÅäÖĘ£¬ŠčÓĆĮæĶ²ĮæČ”___________ml4mol/L CuSO4ČÜŅŗ”£
(3)ŹµŃéŹŅÓĆ4mol/LµÄĮņĖįĶČÜŅŗĻ”ŹĶÅäÖĘČÜŅŗĖłŠčµÄŹµŃé²½ÖčÓŠ£ŗ
ĘäÖŠÕżČ·µÄ²Ł×÷Ė³ŠņĪŖ
¢ŁĶłÉÕ±ÖŠ¼ÓČėŌ¼100mlĖ®½ųŠŠ³õ²½Ļ”ŹĶ£¬ĄäČ“ÖĮŹŅĪĀ
¢ŚÓĆĮæĶ²ĮæČ”Ņ»¶ØĢå»ż4mol/L µÄĮņĖįĶČÜŅŗÓŚŅ»ÉÕ±ÖŠ
¢Ū¼ĘĖćĖłŠč4mol/L ĮņĖįĶČÜŅŗµÄĢå»ż
¢Ü½«ČÜŅŗµßµ¹Ņ”ŌČŗó×Ŗ“ęÓŚŹŌ¼ĮĘæ
¢Ż¼ÓĖ®ÖĮŅŗĆęĄėČŻĮæĘæ1-2cm“¦øÄÓĆ½ŗĶ·µĪ¹Ü½ųŠŠ¶ØČŻ
¢ŽĻ“µÓÉÕ±ŗĶ²£Į§°ō2-3“Ī²¢½«Ļ“µÓŅŗ×¢ČėČŻĮæĘ棬ĒįĒįŅ”¶ÆČŻĮæĘ棬Ź¹ČÜŅŗ»ģŗĻ¾łŌČ
¢ß½«ČÜŅŗ×ŖŅĘČėČŻĮæĘæ
(4)ÅäÖĘČÜŅŗ¹ż³ĢÖŠ£¬Čē¹ū³öĻÖŅŌĻĀĒéæö£¬¶Ō½į¹ūÓŠŗĪÓ°Ļģ£ØĢī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±£©
£Į£®¶ØČŻŗóČūÉĻĘæČū·“ø“Ņ”ŌČ£¬¾²ÖĆŗó£¬ŅŗĆę²»µ½æĢ¶ČĻߣ¬ŌŁ¼ÓĖ®ÖĮæĢ¶ČĻß
B£®¶ØČŻŹ±ø©ŹÓæĢ¶ČĻß
C£®ČŻĮæĘæĪ“øÉŌļ¼“ÓĆĄ“ÅäÖĘČÜŅŗ
£Ø5£©ŹµŃé¹ż³ĢÖŠÓƵ½ÕōĮóĖ®”£ČēĶ¼ĪŖŹµŃéŹŅÖĘČ”ÕōĮóĖ®µÄ×°ÖĆŹ¾ŅāĶ¼”£Ķ¼ÖŠµÄĮ½“¦Ć÷ĻŌµÄ“ķĪóŹĒ ______________________________£»_______________________________”£ŹµŃ鏱AÖŠ³ż¼ÓČėÉŁĮæ×ŌĄ“Ė®Ķā£¬»¹Šč¼ÓČėÉŁĮæ___ ____£¬Ęä×÷ÓĆŹĒ·ĄÖ¹±©·Š”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com