
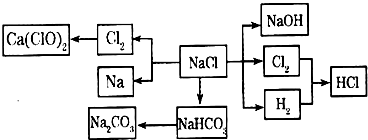
 2HCl���ʴ�Ϊ��H2+Cl2
2HCl���ʴ�Ϊ��H2+Cl2 2HCl��
2HCl�� 2OH-+H2��+Cl2����
2OH-+H2��+Cl2���� 2OH-+H2��+Cl2����
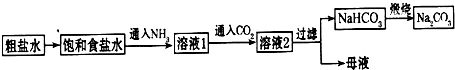
2OH-+H2��+Cl2���� Na2CO3+CO2��+H2O��������������ˮ����������̼����ˮ����ͨ�백������Һ�ʼ��ԣ���ͨ�������̼�����֮�����ת��ΪHCO3-��
Na2CO3+CO2��+H2O��������������ˮ����������̼����ˮ����ͨ�백������Һ�ʼ��ԣ���ͨ�������̼�����֮�����ת��ΪHCO3-�� Na2CO3+CO2��+H2O����ΪNH3��������ˮ����Һ�������ԣ���ͨ��CO2ʹ֮�����ת��ΪHCO3-��
Na2CO3+CO2��+H2O����ΪNH3��������ˮ����Һ�������ԣ���ͨ��CO2ʹ֮�����ת��ΪHCO3-��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ������ѧ2010��2011ѧ��߶���ѧ����ĩͳ����ѧ����(ѡ��) ���ͣ�022
����һ����;�㷺�Ļ���ԭ�ϣ�ͬʱҲ��һ�����ص���Ⱦ������۸�����ǿ�ҵ��°����ã���ҵ��Ϊ�˴�����Cr2O![]() �����Է�ˮ��������ķ�������������ҵ��ˮ�м���������NaCl������ȣ�����Ϊ�缫���е�⣬�Ӷ�ʹ��Һ��pH��������ˮ������ת��Ϊ���ԣ�����һ��ʱ����Cr(OH)3��Fe(OH)3�������ɣ����˻��ճ�������ҵ��ˮ�и��ĺ����������ŷű���
�����Է�ˮ��������ķ�������������ҵ��ˮ�м���������NaCl������ȣ�����Ϊ�缫���е�⣬�Ӷ�ʹ��Һ��pH��������ˮ������ת��Ϊ���ԣ�����һ��ʱ����Cr(OH)3��Fe(OH)3�������ɣ����˻��ճ�������ҵ��ˮ�и��ĺ����������ŷű���
�Իش�
(1)����������NaCl����________
(2)�缫��Ӧʽ������________������________
(3)��Cr2O![]() ��ΪCr3ʮ�����ӷ���ʽΪ________
��ΪCr3ʮ�����ӷ���ʽΪ________
(4)��ҵ��ˮ�����Ա���Ե�ԭ��Ϊ________
(5)�ܷ����ʯī��ͭ�缫________(��ܡ����ܡ�)��ԭ��Ϊ________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com