
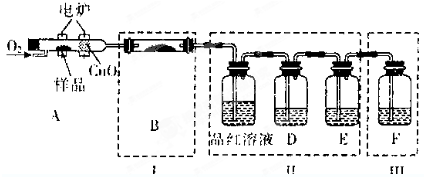
| ||
| ||
| 2.04g |
| 204g/mol |
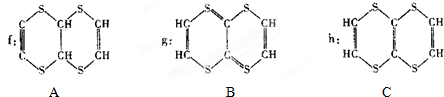
 ��
�� ��
��

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | I1 | I2 | I3 | I4 |
| X | 500 | 4600 | 6900 | 9500 |
| Y | 580 | 1800 | 2700 | 11600 |
| A��Ԫ��X��Y������ͬ����Ԫ�� |
| B��Ԫ��X�������Ǣ�A��Ԫ�� |
| C��Ԫ��X�����γɻ�����ʱ����ѧʽ������XCl |
| D��Ԫ��Y�ڻ�ѧ�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ���� | ʵ�������� | ������ ��ɿ� | �ж����� |
| �� | ȡ������ɫ��Һ���������Ȼ�̼�������ã� | �²���Һ�ʳȺ�ɫ��֤��Br2���ܽ�Fe2+����ΪFe3+�� | �� | �� �������֣� |
| �� | ȡ������ɫ��Һ������ ���ε��۵⻯����Һ�� | ��Һ��Ϊ��ɫ��֤��Br2�� ��Fe2+����ΪFe3+�� | �� | �� ��д���ӷ���ʽ�� |
| �� | ȡ������ɫ��Һ������ ���� �� | ��Һ��Ϊ��ɫ��֤��Br2�� ��Fe2+����ΪFe3+�� | �ɿ� | 1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ij��Һ�м���ϡ���ᣬ����������ͨ�����ʯ��ˮ��ʯ��ˮ����ǣ�����Һһ����̼������Һ |
| B���ò�˿պȡ����ij��Һ������ɫ��Ӧ������ʻ�ɫ������Һ��һ����������Һ |
| C����ijδ֪��Һ�м���ϡNaOH��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�������������ԭ��Һ��û��NH4+ |
| D��ij��Һ�м���BaCl2��Һ������������ϡ����İ�ɫ����������Һһ������Ag+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
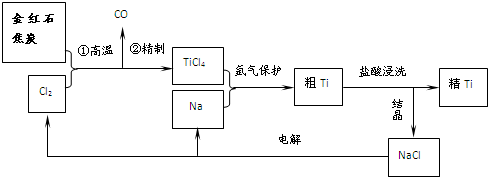
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
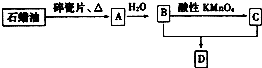 A��һ����Ҫ�Ļ���ԭ�ϣ�A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��D�Ǿ��й�����ζ�����������A��B��C��D��һ�������´�������ת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ���
A��һ����Ҫ�Ļ���ԭ�ϣ�A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��D�Ǿ��й�����ζ�����������A��B��C��D��һ�������´�������ת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
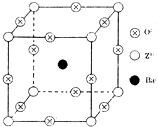 ��֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���48��X��һ��1��1���⻯������м��ЦҼ����Цм���Z�ǽ���Ԫ�أ�Z�ĵ��ʺͻ������й㷺����;����֪Z�ĺ˵����С��28���Ҵ������2��δ�ɶԵ��ӣ���ҵ������ZO2��̼�ᱵ������״̬����ȡ������M��M�ɿ���һ�ֺ������Σ���M�������ġ�ѹ�����ܡ���Ӧ���ڳ������ķ���װ�ã���X���߷�����M�������С�ظ���λΪ�����壨��ͼ�����߳�Ϊ4.03��10-10m������λ��ΪZ4+��ռ������λ��ΪBa2+��ռ����������λ��ΪO2-��ռ��
��֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���48��X��һ��1��1���⻯������м��ЦҼ����Цм���Z�ǽ���Ԫ�أ�Z�ĵ��ʺͻ������й㷺����;����֪Z�ĺ˵����С��28���Ҵ������2��δ�ɶԵ��ӣ���ҵ������ZO2��̼�ᱵ������״̬����ȡ������M��M�ɿ���һ�ֺ������Σ���M�������ġ�ѹ�����ܡ���Ӧ���ڳ������ķ���װ�ã���X���߷�����M�������С�ظ���λΪ�����壨��ͼ�����߳�Ϊ4.03��10-10m������λ��ΪZ4+��ռ������λ��ΪBa2+��ռ����������λ��ΪO2-��ռ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com