
ij����С���������ͼ��ʾװ�ã����ڻ������������ڵ���ǿ�����е������������л������NaCl��Һ�����ʵ��(��ʱ����ֹˮ��a���ر�ֹˮ��b)�����ڴ��ģ�ʵ�鲢δ�ﵽԤ��Ŀ�ģ���Ҳ���������˸��˵�������������Ƿ������ش���������(ͼ�������ӽ���Ĥֻ���������Ӻ�ˮ����ͨ��)��
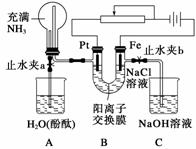
(1)д��Bװ���еĵ缫��Ӧʽ��
Pt��____________________________________________________________________��
Fe��____________________________________________________________________��
(2)д���۲쵽��Aװ���е�����
��________________________________________________________________________��
��________________________________________________________________________��
��________________________________________________________________________��
(3)���۲쵽Aװ���е���������ǹر�ֹˮ��a����ֹˮ��b���ٹ۲�Cװ�á���������˵�����ɣ�����������д���йط�Ӧ����ʽ__________________________
________________________________________________________________________��
(4)����ﵽ���NaCl��Һ��Ŀ�ģ�Ӧ��θĽ�װ�ã������������________________________________________________________________________��
�𰸡�(1)2H����2e��===H2��(��2H2O��2e��===2OH����H2��)��Fe��2e��===Fe2��
(2)��Aװ���ձ��е�H2O��������ƿ��������ɫ��Ȫ������ƿ��Һ��������������ർ�ܿ�һ���̶Ⱥ������������뵼�ܿ���ƽ��������ձ�����Һ�ʺ�ɫ�����ܿ��������ݳ�
(3)Fe2����2OH��===Fe(OH)2����4Fe(OH)2��2H2O��O2===4Fe(OH)3[��4Fe2����8OH����2H2O��O2===4Fe(OH)3��]
(4)��Fe�缫����C��Pt�ȶ��Ե缫(��װ�������缫����λ�õ�)
������(1)��ͼ֪��Pt���������������ӷŵ������������ʵ缫��ӦʽΪ2H����2e��===H2��(��д��2H2O��2e��===2OH����H2��)��Fe��Ϊ�����������õ缫���ʵ缫��ӦʽΪFe��2e��===Fe2����(2)��ֹˮ��a���ر�ֹˮ��bʱ�����ɵ�����������ƿ��ѹǿ��������ѹ���������������ڿ����ų������ձ���ˮ�Ӵ�����ʼ�ܽ⣬����Aװ���ձ��е�H2O��������ƿ������ɫ��Ȫ������������������ˮ������ƿ��Һ��������������ർ�ܿ�һ���̶Ⱥ��������������ܿ���ƽ�������ձ�����Һ�ʺ�ɫ�����ܿ�������(����)�ݳ���(3)�ر�ֹˮ��a����ֹˮ��b��Pt���������������Ὣ�������Һѹ���ձ�C�У�������Ӧ��Fe2����2OH��===Fe(OH)2����4Fe(OH)2��2H2O��O2===4Fe(OH)3[��4Fe2����8OH����2H2O��O2===4Fe(OH)3��]��(4)����ﵽ���NaCl��Һ��Ŀ�ģ�Ӧ��Fe�缫����C��Pt �ȶ��Ե缫��װ�������缫����λ�õȡ�
�ȶ��Ե缫��װ�������缫����λ�õȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʢ������ͭˮ��Һ���Թ�����백ˮ�������γ�������������Ӱ�ˮ���������ܽ�õ�����ɫ������Һ�����жԴ�����˵����ȷ����( )
A����Ӧ����Һ�в������κγ��������Է�Ӧǰ��Cu2+��Ũ�Ȳ���
B����[Cu(NH3)4] 2+�����У�Cu2+�����¶Ե��ӣ�NH3�ṩ�չ��
C����Ӧ�����Һ�����Ҵ�����Һû�з����仯
D�������ܽ����������ɫ���������[Cu(NH3)4] 2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ����ȡ����Ӧ����
A��CH4��Cl2�Ļ��������պ���ɫ��dz B����ϩͨ������KMnO4��Һ�У���Һ��ɫ
C��������ˮ��Ϻ���ˮ����ɫ D����ϩͨ����ˮ�У���ˮ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��F���ֶ�����Ԫ�أ�AԪ�ص�������ɫ��ӦΪ��ɫ��5.8 g B����������ǡ������100 mL 2 mol��L��1������ȫ��Ӧ��Bԭ�Ӻ�������������������ȡ�Fԭ������������F2�ڻ���ɫ����C2��ȼ�ղ�����ɫ���档DԪ��ԭ�ӵ������������Ǵ�����������3������EԪ�صĻ�����������ࡣ�������������ش�
��1��E��Ԫ�ط���Ϊ ��
��2��A��D�γɵ���ɫ������A2D2�������ʽΪ ��
��3��C2��ADF��Һ��Ӧ�����ӷ���ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�������õ�ⷨ����������ˮ�������������������������������ʢ�ź�����ˮ��ԭ��ʾ��ͼ���£�����˵������ȷ���� (����)
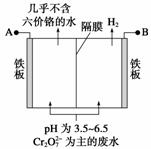
A��AΪ��Դ����
B����������Һ�з�����������ԭ��ӦΪ
Cr2O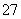 ��6Fe2����14H��===2Cr3����6Fe3��
��6Fe2����14H��===2Cr3����6Fe3�� ��7H2O
��7H2O
C��������������ҺpH����
D����������������ܽ⣬���ռ���H2 13.44 L(��״��)ʱ����0.1 mol Cr2O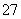 ����ԭ
����ԭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)���н�������Խ��Խ���ױ���ʴ (����)
(2)�����������ں����������ⸯʴԭ��һ�� (����)
(3)���ﻷ���½���������ʴ (����)
(4)Al��Fe��Cu�ڳ�ʪ�Ŀ����и�ʴ������������ (����)
(5)���������绯ѧ��ʴʱ��������ʧȥ��������Fe3�� (����)
(6)�ڽ������渲�DZ����㣬���������������ȫʧȥ�˶Խ����ı�������(����)
(7)��ӵ����������������������˵��أ���������������������ԭ��ء����߾�����Ч�ر������������ױ���ʴ (����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڵ��ԭ����������ȷ���� (����)
A��Ϊ��ֹ�ִ���ʴ�����ִ�����������Դ�ĸ�������
B����пƬ�϶�ͭ�����Ȼ�п��Һ�����Һ
C������Ȼ�����Һ�Ʊ�����������ʱ������������
D���õ�ⷨ����ͭʱ���������Ͽ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�������ʹ�÷�����ʵ�������ȷ����( )
A��ϴ������ƿ������ƿ���ԷŽ������к��
B����ʽ�ζ���װ��Һǰ���������ø���Һ��ϴ
C�����ζ�ʵ���У��ô�����Һ��ϴ��ƿ�Լ�Сʵ�����
D��������ƿ����Һʱ������ˮ�����̶��ߣ������õζ�����������Һ�塣
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com