

 2CH3CHO + 2H2O
2CH3CHO + 2H2O  CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O 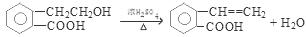
 2CH3CHO + 2H2O���۷�Ӧ�Ļ�ѧ����ʽ�ǣ�CH3CH2OH + CH3COOH
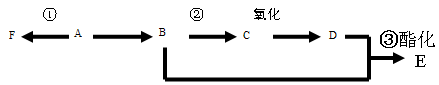
2CH3CHO + 2H2O���۷�Ӧ�Ļ�ѧ����ʽ�ǣ�CH3CH2OH + CH3COOH CH3COOCH2CH3 + H2O����n(H2O) =10.8g��18g/mol=0.6mol������ÿ1mol���Ҵ�ȼ�ղ���3mol��ˮ�������Ҵ������ʵ�����0.2mol. CO��CO2��ˮ��������Ϊ27.6g,����ˮ������Ϊ10.8g,��CO��CO2��������16.8g.����CO��CO2�����ʵ����ֱ���x��y,�����C�غ�ɵ�x+y=0.4.�ṹ�����غ�ɵ�28x+44y=16.8.���x=0.05mol��y=0.35mol������CO��������0.05mol��28g/mol=1.4g������1������B�Ľṹ��ʽ��֪��B�еĺ���������������ȩ�����Ȼ�����2��A��C�ķ�Ӧ��������ȥ��Ӧ��ͬ���칹���Ƿ���ʽ��ͬ���ṹ��ͬ�Ļ������A��D�л�Ϊͬ���칹�����C��D����3����A����C�Ļ�ѧ����ʽ��
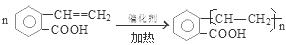
CH3COOCH2CH3 + H2O����n(H2O) =10.8g��18g/mol=0.6mol������ÿ1mol���Ҵ�ȼ�ղ���3mol��ˮ�������Ҵ������ʵ�����0.2mol. CO��CO2��ˮ��������Ϊ27.6g,����ˮ������Ϊ10.8g,��CO��CO2��������16.8g.����CO��CO2�����ʵ����ֱ���x��y,�����C�غ�ɵ�x+y=0.4.�ṹ�����غ�ɵ�28x+44y=16.8.���x=0.05mol��y=0.35mol������CO��������0.05mol��28g/mol=1.4g������1������B�Ľṹ��ʽ��֪��B�еĺ���������������ȩ�����Ȼ�����2��A��C�ķ�Ӧ��������ȥ��Ӧ��ͬ���칹���Ƿ���ʽ��ͬ���ṹ��ͬ�Ļ������A��D�л�Ϊͬ���칹�����C��D����3����A����C�Ļ�ѧ����ʽ��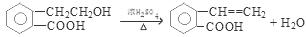 ��4��C��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽ��
��4��C��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽ�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ж���
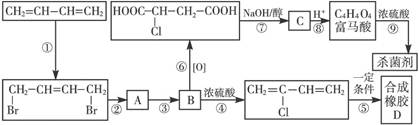
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ | B����ά�� | C�������� | D��������ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
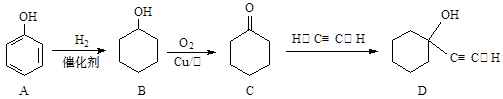
 ����Ӧ���������� ��ѡ����ţ���
����Ӧ���������� ��ѡ����ţ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
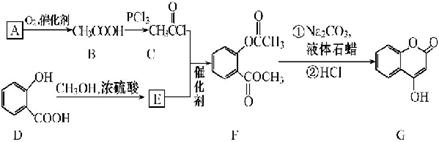
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
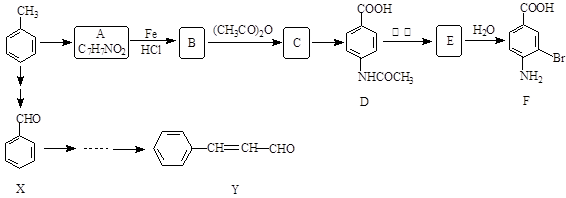
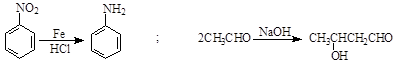 ��
��| A������ʽ��C7H7NO2Br | B�����������ᷴӦ������NaOH��Һ��Ӧ |
| C���ܷ���������Ӧ | D��1 mol F����������2 mol NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
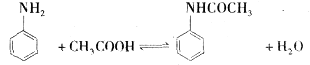
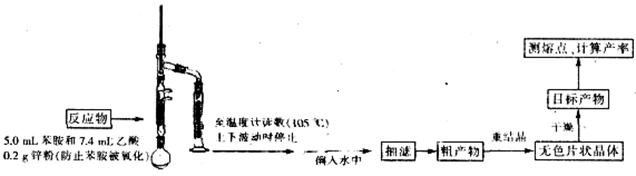
| �Լ����� | ��Է������� | �ܶ�g/ml | �۵�� | �е�� | �ܽ�� |
| ���� | 93 | 1.02 | ��6.2 | 184.4 | ������ˮ�����Ҵ������ѡ������� |
| ���� | 60 | 1.05 | 16.7 | 118 | ����ˮ���Ҵ������ѵ� |
| �������� | 135 | 1.21 | 114��116 | 280��290 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
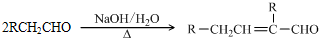
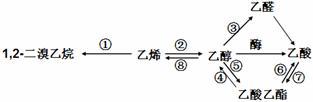
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
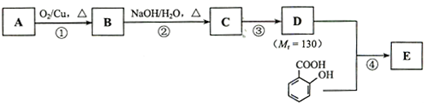
| A����Ӧ���Ǽӳɷ�Ӧ����Ӧ�ۢ�����ȥ��Ӧ�������������ķ�Ӧ����ȡ����Ӧ |
| B����������������NaOH��Һ��Ӧ��ֻ������ |
| C�������ʵ�������ϩ���Ҵ�������������Ӧʱ��������ͬ |
| D��1��2-�������顢��ϩ���Ҵ������ϵ��ⱻ��ȡ������һ��ȡ�����ﶼ��һ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com