

 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O�� CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O�� 3Cu+N2+3H2O��
3Cu+N2+3H2O�� 3Cu+N2+3H2O��
3Cu+N2+3H2O�� CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
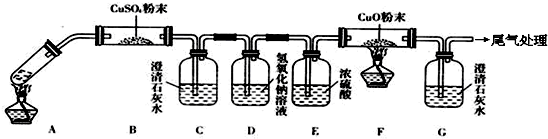
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д��������������
ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д���������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��������������е�����ѧ������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��14�֣�ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ�����Ѿõ�С�մ���Ʒ�д��������������
��1������һ����ȡһ����������Ʒ�����������м��������غ���ȴ������ʣ��������������㡣ʵ���м��������ص�Ŀ���� ��
��2������������ȡһ������Ʒ������С�ձ��У�������ˮ�ܽ⣬��С�ձ��м��������Ȼ�����Һ������ϴ�ӣ���������������������������㣺
�ٹ��˲����У������ձ���©����õ��IJ���������______________________��
���������жϳ����Ƿ���ȫ�ķ�����_______________________________________
���������Լ���Ϊ������������֪�Ƶ���Ʒ9.5g������ij�������Ϊ19.7g������Ʒ��̼���Ƶ���������Ϊ_________________������һλС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��������������и�����ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��14�֣�ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ�����Ѿõ�С�մ���Ʒ�д��������������
��1������һ����ȡһ����������Ʒ�����������м��������غ���ȴ������ʣ��������������㡣ʵ���м��������ص�Ŀ���� ��
��2������������ȡһ������Ʒ������С�ձ��У�������ˮ�ܽ⣬��С�ձ��м��������Ȼ�����Һ������ϴ�ӣ���������������������������㣺
�ٹ��˲����У������ձ���©����õ��IJ���������______________________��
���������жϳ����Ƿ���ȫ�ķ�����_______________________________________
���������Լ���Ϊ������������֪�Ƶ���Ʒ9.5g������ij�������Ϊ19.7g������Ʒ��̼���Ƶ���������Ϊ_________________������һλС������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com