
B�� [ʵ�黯ѧ]
���������Ƽ�ȩ(NaHSO2��HCHO��2H2O)��ӡȾ��ҽҩ�Լ�ԭ���ܹ�ҵ��Ӧ�ù㷺����Na2SO3��SO2��HCHO ��п��Ϊԭ���Ʊ����������Ƽ�ȩ��ʵ�鲽�����£�
����1������ƿ��(װ����ͼ ��ʾ) ����һ����Na2SO3��ˮ�������ܽ⣬����ͨ��SO2������ҺpH ԼΪ4���Ƶ�NaHSO3��Һ������2����װ��A �е����ܻ�����Ƥ��������ƿ�м����Թ�����п�ۺ�һ������ȩ��Һ����80 ~ 90���£���ӦԼ3h����ȴ�����£����ˡ�����3������Һ�������Ũ������ȴ�ᾧ��
(1)װ��B ���ձ���Ӧ�������Һ�� ��
(2)�ٲ���2 �У���Ӧ���ɵ�Zn(OH)2�Ḳ����п�۱�����ֹ��Ӧ���У���ֹ���������Ĵ�ʩ��
�����������л�������Ҫ���ʳ�H2O ��� (�ѧʽ)��
(3)�ٳ���װ������������������ѹϵͳ��� �� (����������)������������Ҫ�ɷ��� �� (�ѧʽ)��
(4)���������Ƽ�ȩ����ǿ��ԭ�ԣ�����120�����Ϸ����ֽ⡣����3 �в��ڳ�������������Ũ����ԭ���� ��
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������壺H2��Cl2��HCl��NH3��NO��H2S��SO2������ͼ��ʾ��װ�ý���ʵ�飬������пհף�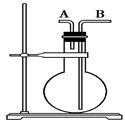
��1����ƿ����ʱ����A�ڽ������ռ���������________����B�ڽ������ռ���������______________��
��2����ƿ�г���ˮʱ������������___________������������
��3������ƿ��װ��ϴҺ������ϴ��ʱ������Ӧ��________�ڽ�����ƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(13��)����������������۷���Ϣ���ʵijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧװ��ʾ��ͼ���й��������£�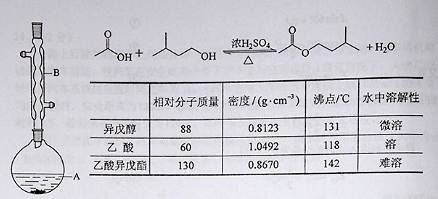
ʵ�鲽�裺
��A�м���4.4 g�����촼��6.0 g�����ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50���ӣ���ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮ����þ���壬����Ƭ�̣����˳�ȥ����þ���壬�������������ռ�140��143 ����֣�������������3.9 g���ش��������⣺
��1��װ��B�������ǣ�
��2����ϴ�Ӳ����У���һ��ˮϴ����ҪĿ���ǣ� �� �ڶ���ˮϴ����ҪĿ���ǣ� ��
��3����ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ�� �����ţ���
A��ֱ�ӽ������������ӷ�Һ©���Ͽڵ���
B��ֱ�ӽ������������ӷ�Һ©���¿ڷų�
C���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
D���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڷų�
��4����ʵ���м�����������Ŀ���ǣ�
��5��ʵ���м���������ˮ����þ��Ŀ���ǣ�
��6������������У�����ѡ��װ����ȷ���ǣ� (����)
��7����ʵ��IJ����ǣ�
A��30�G B��40�G C��50�G D��60�G
��8���ڽ����������ʱ������130 �濪ʼ�ռ���֣�����ƫ (����ߵ�)ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��13�֣������Ǹ�ˮ����([Cr(CH3COO)2)]2��2H2O�����ɫ���壩��һ���������ռ���ͨ���Զ�������Ӵ��ڣ���������ˮ���ѣ����ڴ������������ᡣʵ�����Ʊ������Ǹ�ˮ�����װ����ͼ��ʾ���漰�Ļ�ѧ����ʽ���£�
Zn(s) + 2HCl(aq) = ZnCl2(aq) + H2(g)
2CrCl3(aq) + Zn(s)= 2CrCl2 (aq) + ZnCl2(aq)
2Cr2+(aq) + 4CH3COO-(aq) + 2H2O(l) = [Cr(CH3COO)2]2��2H2O (s)
��ش��������⣺
��1����������װ�������Եķ����� ��
��2����������ҺӦ����װ�� �У���дװ�ñ�ţ���ͬ��������Ӧ����װ�� �У�
װ��4�������� ��
��3����ʵ��������������Һ��ˮ����У���ԭ���� ��
��4�������ɵ�CrCl2��Һ��CH3COONa��Һ���ʱ�IJ����� ����A�� ����B ������رա�����
��5����ʵ����п�����������ԭ���� ��
��6��Ϊϴ��[Cr(CH3COO)2)]2��2H2O��Ʒ�����з��������ʺϵ��� ��
A����������ϴ��������ˮϴ B��������ˮϴ�������Ҵ�ϴ
C��������ˮϴ����������ϴ D�������Ҵ�ϴ�ӣ���������ϴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��12�֣���Ȼˮ����������ˮ����Ҫ��Դ������Ȼˮ��ÿ������õ�ˮһ���뾭�����������ɱ�������Ȳ��衣
��1������������Ҫ��ˮ�м������������罫���μ���ˮ���ܴﵽ��ˮĿ�ģ�
ԭ���� �������ӷ���ʽ��ʾ����
��2����������������ˮɱ����������������ӷ���ʽ���������� ��
��3������ˮ������������� (K2FeO4)����ǿ���������ú��������á���ҵ�Ͽ�ͨ�����������Ʊ�������أ�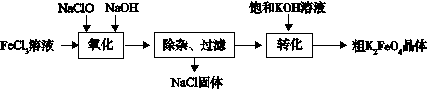
��������:�������������Ի�������Һ�л��ֽ⣬�ڼ�����Һ���ȶ���
��ɡ������������е����ӷ���ʽ
��Fe3+ + ��ClO- +�� ="��" FeO42- + ��Cl- + ��
��ת����������ʵ����Na2FeO4�Ƶ�K2FeO4�������ö��� �ԵIJ�ͬ��
�۽��������ɴ�K2FeO4������ᴿ�����ֲ�Ʒ�� �ܽ⣬Ȼ���ټ��뱥��KOH��Һ����ȴ�ᾧ�����ˡ�
�ܸ�����ص�Ӧ�û��ڲ�����չ�С�����Ƴɸ�����أ� ��ط�ӦΪ��
3Zn + 2K2FeO4 + 8H2O  3Zn(OH)2 + 2Fe(OH)3 + 4KOH
3Zn(OH)2 + 2Fe(OH)3 + 4KOH
�ŵ�ʱ��������ӦΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧʵ��С���ͬѧΪ̽���ͱȽ�SO2����ˮ��Ư���ԣ���������µ�
ʵ��װ�á�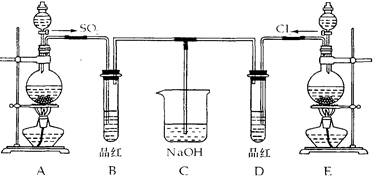
��1��ʵ������װ��A�Ʊ�SO2ijͬѧ��ʵ��ʱ���ִ�A�ķ�Һ©��������©����Һ��δ
���£�����Ϊԭ������ǣ�
��2��ʵ������װ��E�Ʊ�Cl2�䷴Ӧ�Ļ�ѧ��ѧ����ʽΪ��MnO2+4HCl��Ũ��=C12��+MnCl2+2H2O
Ũ���������Ϊ��
��3����Ӧ��ʼһ��ʱ��۲쵽 B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�
B�� D ��
��ֹͣͨ�����ٸ�B��D�����Թֱܷ���ȣ������Թ��е�����ֱ�Ϊ
B�� D ��
��4����һ��ʵ��С���ͬѧ��ΪSO2����ˮ����Ư���ԣ�����Ϻ��Ư���Կ϶����ǿ�����ǽ��Ƶõ�SO2��CI2��1��1ͬʱͨ�뵽Ʒ����Һ�У����������ɫЧ���������������������������������ԭ���û�ѧ����ʽ��ʾ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
CaCO3�㷺��������Ȼ�磬��һ����Ҫ�Ļ���ԭ�ϡ�����ʯ��Ҫ�ɷ�ΪCaCO3�������������ĺ�����(��FeS��)��ʵ�����ô���ʯ��ϡ���ᷴӦ�Ʊ�CO2���塣����װ�ÿ�����CO2������ᴿ���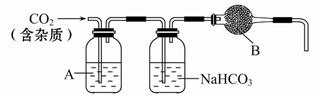
���������գ�
(1)��Ũ��������1��1(�����)��ϡ����(Լ6 mol��L-1)��Ӧѡ�õ�������_____��
a.�ձ�������b.������������c.��Ͳ������d.����ƿ
(2)����װ���У�A��_____��Һ��NaHCO3��Һ��������_____��
(3)����װ���У�B������_____�������ʵ��õ��������ռ������������ⶨCO2�ķ����������B����ʧЧ���ⶨ���_____(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
(4)һ���Է�����ʯ��(������)��CaCO3��ʳ���е��ܳ��������۷���������ָ��֮һ���ⶨ�ܳ�������Ҫʵ�鲽��������£�
���顢���ء������ܽ�����ˡ�������ɡ���ȴ�����ء����أ�Ϊ�˽�ʯ���ܳ���Ӧѡ�õ��Լ���_____��̼����ܳ���Ӧѡ�õ��Լ���_____��
a.�Ȼ�����Һ��������b.ϡ����
c.ϡ���ᡡ����������d.������
(5)���ܳ����ⶨʵ���У�Ϊ�˻��ʯ����̼��Ƶ�����ܳ�����Ӧ���ܳ�_____��ԭ����_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
K3[Fe(C2O4)3]��3H2O[���������(��)��ؾ���]������ˮ,�������Ҵ�,����Ϊ�л���Ӧ�Ĵ�����ʵ���ҿ�����мΪԭ���Ʊ�,��ط�Ӧ�Ļ�ѧ����ʽΪ:Fe+H2SO4 FeSO4+H2��
FeSO4+H2��
FeSO4+H2C2O4+2H2O FeC2O4��2H2O��+H2SO4
FeC2O4��2H2O��+H2SO4
2FeC2O4��2H2O+H2O2+H2C2O4+3K2C2O4 2K3[Fe(C2O4)3]+6H2O
2K3[Fe(C2O4)3]+6H2O
2Mn +5C2
+5C2 +16H+
+16H+ 2Mn2++10CO2��+8H2O
2Mn2++10CO2��+8H2O
�ش���������:
(1)��м�г�����Ԫ��,������Ʊ�FeSO4ʱ������ж���H2S����,�������������������Һ���ա���������װ����ȷ���������������� 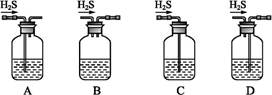
(2)�ڽ�Fe2+�����Ĺ�����,�������Һ�¶Ȳ�����40 ��,��������������������;�õ�K3[Fe(C2O4)3]��Һ��,�����Ҵ���������������������������
(3)�����������ᾧˮ��ͨ�������������ⶨ,��Ҫ������:�ٳ���,�����ں������ѽᾧˮ,����ȴ,�ܳ���,����������������������(�����˲�����),���㡣�������δ�ڸ������н���,��õľ����������ᾧˮ������������(�ƫ�ߡ���ƫ�͡�����Ӱ�족);����ݵ�Ŀ������������������������������������
(4)������C2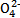 �����IJⶨ��������KMnO4����Һ�ζ�����ȡ���������(��)��ؾ���m g����ˮ���250 mL��Һ,ȡ��20.00 mL������ƿ��,��0.010 0 mol��L-1�ữ�ĸ��������Һ���еζ���
�����IJⶨ��������KMnO4����Һ�ζ�����ȡ���������(��)��ؾ���m g����ˮ���250 mL��Һ,ȡ��20.00 mL������ƿ��,��0.010 0 mol��L-1�ữ�ĸ��������Һ���еζ���
�����в�����˵����ȷ������������
A.�ζ���������ˮϴ�Ӻ�,����װ�����Һ
B.װ�����Һ��,�ѵζ��ܼ��ڵζ��ܼ���,����ת������,�ų�������Һ,ʹ�������Һ��
C.�ӽ��յ�ʱ,��������ˮ��ϴƿ�ں͵ζ��ܼ�����ҵ�Һ��
����ͬѧ��Ϊ�õζ����̲���Ҫָʾ��,��ô�ζ��յ������Ϊ����������������,���ﵽ�ζ��յ����ĸ��������ҺV mL,��ô����������C2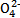 ����������Ϊ��������(�ú�V��m��ʽ�ӱ�ʾ)��
����������Ϊ��������(�ú�V��m��ʽ�ӱ�ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪��P2O5�������������Ҵ����Ʊ���ϩ����Ӧ�¶�Ϊ80��~210�档ij�о���С����������µ�װ���Ʊ��������������ϩ���壨�гֺͼ���������ȥ����

��1������a������Ϊ_________________
��2���û�ѧ��Ӧ����ʽ��ʾ�����Ʊ���ϩ��ԭ��______________________________��
��3����֪P2O5��һ�����Ը��������ˮ�Ŵ����ȣ���ʵ�������P2O5���Ҵ��ܷ������ã���Ӧ�����IJ�ͬ�������ɲ�ͬ�������������Ǿ�Ϊ������ˮ�����ʣ��е�ϵ͡�д���Ҵ������ᷴӦ��������������Ļ�ѧ����ʽ�������ýṹʽ��ʾΪ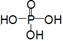 ��__________________________________��
��__________________________________��
��4��ijͬѧ��Ϊ������װ����֤��������ϩ�������ܣ�������___________________��
��5��ijͬѧ������֪��40%����ϩ��������ʽΪC2H6ClO3P����Һ��NaOH�����Ϳɿ��ٲ���ˮ�������õ���ϩ�������������߿��ڻ�������ϩ����Һ��NaOH������ȡ��ϩ��װ�ü�ͼ(�г�������)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com