
 4Al+3O2���������ʯ������:_______________________________��
4Al+3O2���������ʯ������:_______________________________��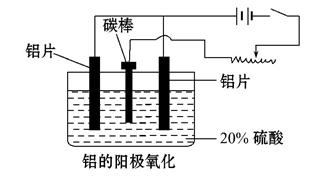



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
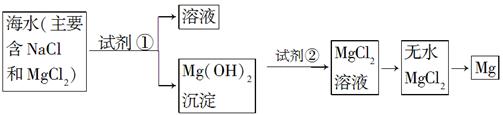
| ���� | �Ƿ���ȷ | �������� |
| ����1��ֱ������ˮ�м�������� | ����ȷ | ��һ�� |
| ����2�����¼���������ˮ���ټ�������� | ������ | ������ |
| ����Ϊ����������������ǣ����������ģ� | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��̼�� | B��ϡ���� | C������������Һ | D����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 4AlCl3(g)��3O2(g)����H>0 ��������������(����)
4AlCl3(g)��3O2(g)����H>0 �����з����������(����)| A������Ӧ��ϵ��ѹǿ����Ӧ���ʿ��ܼӿ� |
| B������̼�ۣ�ƽ�������ƶ���ԭ����̼��O2��Ӧ���������������Ũ���ҷų����� |
| C��������ڵ�Al2O3��AlCl3��Һ���ܵõ������� |
| D����AlCl3��6H2O���Ȼ��������м��ȣ�Ҳ���Ƶ���ˮ�Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
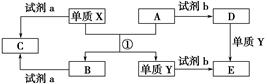
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 xFe2O3�������Ʊ�Al2(S04)3
xFe2O3�������Ʊ�Al2(S04)3 18H2O�������������£�
18H2O�������������£�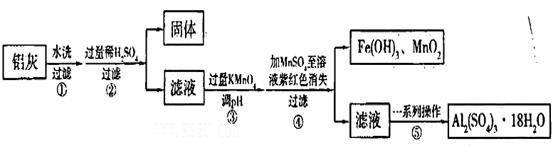
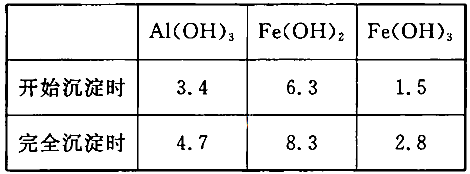
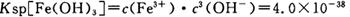 ��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��
��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��| A�������� | B������ | C�������� | D���ƾ���E��©�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��һ�� | ����0.5 gˮ | �ܿ������ |
| �ڶ��� | ����1 g���� | Լ��30 s������ |
| ������ | ����1 g��ˮ����ͭ | 1 minʱ��û�����Է�Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ȫ�� | B���٢ڢ� |
| C���٢ڢۢ� | D���ڢۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com