
���������������ҩ�Ϳ�ϵ�����Ӧ�ù㷺��
(1)���й��ڻ�������˵������ȷ����________��
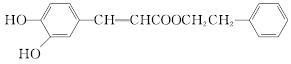
��
A����FeCl3��Һ��������ɫ
B���ɷ���������Ӧ��������Ӧ
C�������巢��ȡ����Ӧ
D��1 mol ��������������2 mol NaOH��Ӧ
(2)��Ӧ����һ����ϩ��ֱ���Ʊ������������·�����
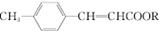
 ��2ROH��2CO��O2
��2ROH��2CO��O2 2 ��2H2O
2 ��2H2O
��
�������ķ���ʽΪ________��1 mol�����������________mol H2ǡ����ȫ��Ӧ���ɱ���������
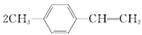
(3)���������ɷ����廯��������ֱ�ͨ����ȥ��Ӧ��ã���ֻ�Т�����Na��Ӧ����H2����Ľṹ��ʽΪ________(дһ��)���ɢ����ɢ�ķ�Ӧ����Ϊ________��
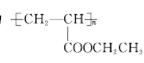 |
(4)�ۺ��� �������Ʊ�Ϳ�ϣ��䵥��ṹ��ʽΪ
________________���������Ʒ�Ӧ�ٵķ�����������ϩΪ�л���ԭ�Ϻϳɸõ��壬�漰�ķ�Ӧ����ʽΪ____________________________________��
(1)AC��(2)C9H10��4
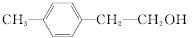 |
(3) �� ��
NaOH�Ĵ���Һ������
(4)CH2===CHCOOCH2CH3
CH2===CH2��H2O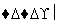 CH3CH2OH
CH3CH2OH
2CH2===CH2��2CH3CH2OH��2CO��O2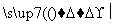 2CH2===CHCOOCH2CH3��2H2O
2CH2===CHCOOCH2CH3��2H2O
[����] (1)�û������к��з��ǻ�����A����ȷ��������û��ȩ�������ܷ���������Ӧ��B��������ڷ��ǻ�����λ�Ͷ�λ�϶���Hԭ�ӣ��������巢��ȡ����Ӧ�������е�̼̼˫�������巢���ӳɷ�Ӧ��C����ȷ����������е�2�����ǻ���1������������NaOH��Ӧ�����������3 mol NaOH��D����
(2)���ݻ������Ľṹ��ʽ��֪�����ʽΪC9H10����������б�����̼̼˫��������H2�����ӳɷ�Ӧ����1 mol�û���������4 mol H2�����ӳɷ�Ӧ��
(3)�����������к���̼̼˫������˫�����ɴ���±����������ȥ��Ӧ�õ���������Na��Ӧ����H2����±�������ܣ��ʻ������Ľṹ��ʽΪ
 | |||
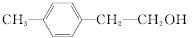 | |||
��
±�������������ƵĴ���Һ������ʱ������ȥ��Ӧ��
(4)�ɾۺ���Ľṹ��ʽ��֪Ϊ�Ӿ۲�����䵥��ΪCH2===CHCOOCH2CH3���ϳ��л����ȡ���Ʒ�����Ϸ�Ӧ�ٵ�ԭ������Ӧ�ﻹ��Ҫ�Ҵ������������ϩ�ϳ��Ҵ�����CH2===CH2��H2O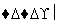 CH3CH2OH��Ȼ���Ϸ�Ӧ�ٵ���Ϣ�����÷�Ӧ
CH3CH2OH��Ȼ���Ϸ�Ӧ�ٵ���Ϣ�����÷�Ӧ
2CH2CH2��2CH3CH2OH��2CO��O2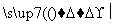 2CH2CHCOOCH2CH3��2H2O���ϳɡ�
2CH2CHCOOCH2CH3��2H2O���ϳɡ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ϣ���������ϡ��͡���Դ������Ϊ�¿Ƽ�����������֧���������й���Ѷ�������(����)
A���ڼ�������������Դʱ�������ܡ�̫���ܡ����ܽ���Ϊ��Ҫ��Դ
B��Ŀǰ�����յȹ����յ��մɷ������������������˷�����������ת��Ч��
C��2002��12��30�շ���ɹ��ġ������ĺš���ʹ���˴����ĸ��ϲ���
D���ϳɸ߷��Ӳ��ϵĹ㷺Ӧ���ǡ��а�������һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ᾧõ���Ǿ���ǿ��õ�����������ϣ��������з�Ӧ·�ߺϳ�(���ַ�Ӧ������ȥ)��

(1)A�������________������Cl2��Ӧ����A��������________��B�еĹ�������________��
(2)��Ӧ�۵Ļ�ѧ����ʽΪ____________________��
(3)��֪��B ���״���������أ���Ӧ·�ߢٵõ��IJ����ˮ��ȡ����Һ���ܳ�ȥ�ĸ�������________��
���״���������أ���Ӧ·�ߢٵõ��IJ����ˮ��ȡ����Һ���ܳ�ȥ�ĸ�������________��
 | |||
 | |||
 (4)��֪�� ��
(4)��֪�� �� 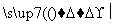 ��H2O����Ӧ·�ߢڵõ�һ�ָ������˴Ź���������4��壬�������շ�����֮��Ϊ________��
��H2O����Ӧ·�ߢڵõ�һ�ָ������˴Ź���������4��壬�������շ�����֮��Ϊ________��
(5)G��ͬ���칹��L��FeCl3��Һ��ɫ��������������ˮ��Ӧδ����ɫ������������L��NaOH���Ҵ���Һ���ȣ������л���Ľṹ��ʽΪ________��(ֻдһ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��һ���л��ϳ��м��壬��ṹ��ʽΪ
 |
��A�ĺϳ�·����ͼ��ʾ������B��H�ֱ����һ���л��
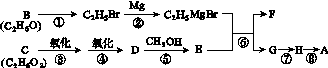
��֪��
 | |||
 | |||

R��R�����������
��ش��������⣺
(1)A��̼ԭ�ӵ��ӻ����������____________��A������(ϵͳ����)��__________________________���ڢಽ��Ӧ��������________��
(2)�ڢٲ���Ӧ�Ļ�ѧ����ʽ��________________��
(3)C������CH2C(CH3)COOH�����ʵ���֮��1��1��Ӧ������ᆳ�Ӿ۵õ����������۾��ľ�Ƭ���Ϣ�Ľṹ��ʽ��________��
(4)�ڢ���Ӧ�Ļ�ѧ����ʽ��________________��
(5)д��������Ԫ������һ��ȡ����ֻ��2��(�����������칹)��A��ͬ���칹��Ľṹ��ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС���Ա�����ϩΪ��Ҫԭ�ϣ���������·�ߺϳ�ҩ����³����
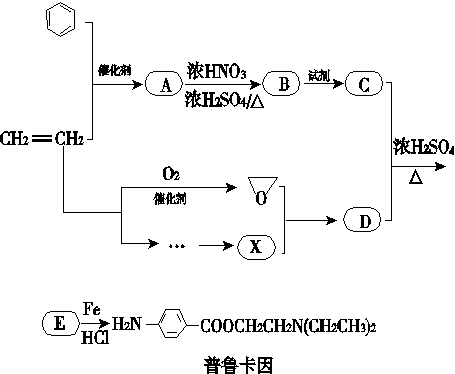
��֪��
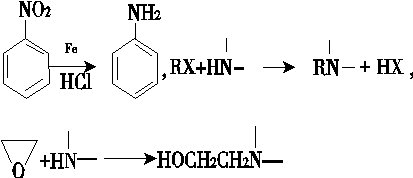
��ش��������⣺
(1)������³��������˵����ȷ����________��
A������Ũ�����γ���
B���������������ӳɷ�Ӧ
C���ɷ���ˮ�ⷴӦ
D���������
(2)д��������B�Ľṹ��ʽ��________��
(3)д��B�D��C��Ӧ������Լ���________��
(4)д��C��D�D��E�Ļ�ѧ��Ӧ����ʽ��________________________________________��
(5)д��ͬʱ��������������B������ͬ���칹��Ľṹ��ʽ��____________________��
�ٷ����к����Ȼ�
��1HNMR����ʾ�����к��б������ұ����������ֲ�ͬ��ѧ��������ԭ��
(6)ͨ��������ϩΪԭ���Ƶû����������X��Ӧ�ϳ�D�����û�ѧ��Ӧ����ʽ��ʾ����ϩΪԭ���Ʊ�X�ĺϳ�·��(�����Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[��ѧ����ѡ��5���л���ѧ����)
������(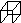 )���и߶ȶԳ��ԡ��������ԡ��������ܼ����ȶ��Ե��ص㣬��˺ϳ������鼰���������Ϊ��ѧ���ע���ȵ㡣��������������������һ�ֺϳ�·�ߣ�
)���и߶ȶԳ��ԡ��������ԡ��������ܼ����ȶ��Ե��ص㣬��˺ϳ������鼰���������Ϊ��ѧ���ע���ȵ㡣��������������������һ�ֺϳ�·�ߣ�
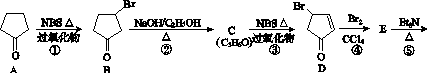
�ش��������⣺
(1)C�Ľṹ��ʽΪ______________��E�Ľṹ��ʽΪ______________��
(2)�۵ķ�Ӧ����Ϊ______________���ݵķ�Ӧ����Ϊ______________��
(3)������A���ɻ����龭������Ӧ�ϳɣ�

��Ӧ1���Լ�������Ϊ________����Ӧ2�Ļ�ѧ����ʽΪ________________________________________����Ӧ3���õ��Լ�Ϊ________��
(4)��I�ĺϳ�·���У���Ϊͬ���칹��Ļ�������________(��������)��
(5)I���ʯ�ҹ��ȿ�ת��Ϊ�����顣������ĺ˴Ź�����������________���塣
(6)�����龭�����ɵõ������������飬����ܵĽṹ��________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ԭ�ӵ�������a g��12Cԭ�ӵ�������b g����NAֻ��ʾ�����ӵ���������ֵ��������˵������ȷ����
�ٸ���ԭ�ӵ����ԭ������Ϊ ����m g����ԭ�ӵ����ʵ���Ϊ
����m g����ԭ�ӵ����ʵ���Ϊ mol���۸���ԭ�ӵ�Ħ��������aNA g����a g����ԭ�������ĵ�����Ϊ16NA
mol���۸���ԭ�ӵ�Ħ��������aNA g����a g����ԭ�������ĵ�����Ϊ16NA
A���٢ۡ��������������� B���ڢ�
C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ��������Һ̬������XY2���״���µ�һ��������O2ǡ����ȫ��Ӧ����ѧ����ʽΪ��XY2(l)��3O2(g)===XO2(g)��2YO2(g)����ȴ���ڱ�״���²��������������672 mL���ܶ���2.56 g��L��1����
(1)��ӦǰO2�������________��
(2)������XY2��Ħ��������________��
(3)��XY2������X��Y��Ԫ�ص���������3��16����X��Y��Ԫ�طֱ�Ϊ________��________(дԪ�ط���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
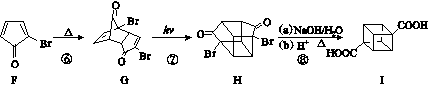
A��G�Ǽ������ķ������ģ��(��ͼ)���ݴ˻ش��������⣺

(1)�����º�̼����ߵ���̬����________(����ĸ)��
(2)�ܹ������ӳɵ�����________�֡�
(3)һ±��������������________(��д��ĸ)��
(4)д��ʵ������D�Ļ�ѧ����ʽ______________________ __________________________________________________��
(5)д��F����������Ӧ�Ļ�ѧ����ʽ_____________________ ___________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com