
�̷�(FeSO4·7H2O)���������������ʵ�����Ͽ��Ƶ�Ħ���ξ��壬��Ӧԭ��Ϊ(NH4)2SO4��FeSO4��6H2O===(NH4)2SO4·FeSO4·6H2O���������̿ɱ�ʾΪ
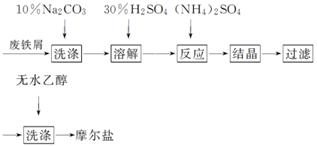
(1)ϴ����Na2CO3����Ҫ������______________________________________��
(2)�ᾧ������Ҫ���������ܼ���Ũ���ᾧ��Ӧ���ȵ�________ʱ��ֹͣ���ȡ�
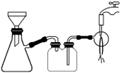
(3)����������ͼ��ʾװ�ý��еģ����ֹ��˸���ͨ������ȣ����˹����ٶȿ��⣬����һ���ŵ���________________________________________________________________________��
(4)����ˮ�Ҵ�ϴ�ӵ�Ŀ����____________________________________________
________________________________________________________________________��
(5)��Ʒ��Fe2���Ķ����������Ƶõ�Ħ������Ʒ���������м�������Fe3����Ϊ�˲ⶨĦ���β�Ʒ��Fe2���ĺ�����һ�����������������KMnO4����Һ�ζ��ķ�������ȡ4.0 g��Ħ������Ʒ������ˮ������������ϡ���ᡣ��0.2 mol·L��1 KMnO4��Һ�ζ�������Һ��Fe2��ȫ��������ʱ������KMnO4��Һ10.00 mL��
�ٱ�ʵ���ָʾ����________(����ĸ)��
A����̪ B������ C��ʯ�� D������Ҫ
�ڲ�Ʒ��Fe2������������Ϊ______________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���ú��ʯ�͡���Ȼ������Դ��˵����ȷ����(����)
A��ʯ���ѽ�õ��������Ǵ�����
B��ʯ�Ͳ�Ʒ�������ھۺϷ�Ӧ
C����Ȼ����һ�����Ļ�ʯȼ��
D��ˮú����ͨ��ú��Һ���õ�������ȼ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�й�ˮ�������е����ã�����˵����ȷ���ǣ� ��
 A��ˮ��һ�ֺܺõ��ܼ� B��ˮ��������û�е������µ�����
A��ˮ��һ�ֺܺõ��ܼ� B��ˮ��������û�е������µ�����
 C������ˮԽ����Խ�� D��û����Ⱦ��ˮ���Ǵ���ˮ
C������ˮԽ����Խ�� D��û����Ⱦ��ˮ���Ǵ���ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��������ˮPH 5.6��ѡ��>������<������=�����룩��������Ϊ��ˮ���ܽ���
 ����һ�������������Ҫ�����ʣ���ɵġ�
����һ�������������Ҫ�����ʣ���ɵġ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������(Na2S2O3)�����������ƺ����ͨ�����Ϸ�Ӧ�Ƶã�Na2SO3��S��,Na2S2O3����������Һ����������ΪNa2S2O3·5H2O��Na2S2O3·5H2O��40��45 ���ۻ���48 ��ֽ⣻Na2S2O3������ˮ���������Ҵ�����ˮ���й����ʵ��ܽ��������ͼ1��ʾ��
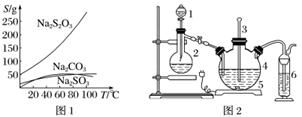
��.�ְ����·����Ʊ�Na2S2O3·5H2O��
�����ƺ�̼���ư���ӦҪ�����һ������������ƿ�У�ע��150 mL����ˮʹ���ܽ⣬�ڷ�Һ©���У�ע��Ũ���ᣬ��װ��2�м����������ƹ��壬����ͼ2���Ӻ�װ�á�
(1)����2������Ϊ________________��װ��6�пɷ���________________(����ĸ)��
A��BaCl2��Һ
B��ŨH2SO4
C������KMnO4��Һ
D��NaOH��Һ
(2)��Һ©��������ע��Ũ����ʹ��Ӧ�����Ķ�����������Ͼ��ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У����ô������������������ȣ���Ӧԭ��Ϊ
��Na2CO3��SO2===Na2SO3��CO2
��Na2S��SO2��H2O===Na2SO3��H2S
��2H2S��SO2===3S����2H2O
��Na2SO3��S��,Na2S2O3
�ܷ�ӦΪ2Na2S��Na2CO3��4SO2===3Na2S2O3��CO2
����SO2�����ͨ�룬������Һ���д���dz��ɫ��������������ͨSO2���壬��ӦԼ��Сʱ��������ʧ������ֹͣͨ���ͼ��ȡ�������ͨ��SO2���ֻ�����dz��ɫ������д����ʱ������Ӧ�����ӷ���ʽ��________________________________________________��
��.����Na2S2O3·5H2O���ⶨ������
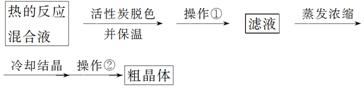
(1)Ϊ���ٲ�Ʒ����ʧ��������Ϊ________���������dz���ϴ�Ӹ������ϴ�Ӳ�������________________(���Լ�)��ϴ�Ӽ���
(2)����Ũ����Һֱ����Һ����ɫ����Ϊֹ������ʱΪʲôҪ�����¶Ȳ��˹���________________________________________________________________________��
(3)�ƵõĴ־��������������������ʡ�Ϊ�˲ⶨ�ֲ�Ʒ��Na2S2O3·5H2O�ĺ�����һ�������������������KMnO4��Һ�ζ��ķ���(�ٶ��ֲ�Ʒ������������KMnO4��Һ����Ӧ)��KMnO4��ҺӦ����________(���ʽ����ʽ��)�ζ����С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У����ڿ������ȶ�������� �� ��
A��NaOH���� B��ϡH2SO4 C��Na2SO3���� D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�����ӷ���ʽ��ȷ���� ( )
A����Ƭ������������Һ��Ӧ��Al��2OH����AlO2��+H2��
B��Cl2ͨ����ˮ�У�Cl2��H2O��Cl����ClO����2H��
C��ͭ��ϡ���ᷴӦ��3Cu��2NO3��+8H����3Cu2����2NO��+4H2O
D��С�մ���Һ�������ʯ��ˮ��Ӧ��HCO3����OH����CO32����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��B������A��Ӧ���ɴ̼�������C ��C����һ�������±�����ΪD��D����ˮ������B ��C�������Һ��Ӧ����A �����������������ʵĻ�ѧʽ�����ǣ�
A B C D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ܱ�������A��B��Ӧ����C���䷴Ӧ���ʷֱ���v��A����v��B����v��C����ʾ����֪v��A����v��B����v��C��֮�������¹�ϵ2v��B��=3v��A����
3v��C��=2v��B������˷�Ӧ�ɱ�ʾΪ�� ����
A.2A+3B=2C B.A+3B=2C C.3A+B=2C D.A+B=C
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com