
 ¢ÜCH3COONH4
¢ÜCH3COONH4 ”£³£ĪĀĻĀ£¬ĪļÖŹµÄĮæÅضČĻąĶ¬µÄ¢Ł”¢¢Ś”¢¢ŪČÜŅŗpH“óŠ”Ė³ŠņĪŖ£ØĢīŠņŗÅ£© > >
”£³£ĪĀĻĀ£¬ĪļÖŹµÄĮæÅضČĻąĶ¬µÄ¢Ł”¢¢Ś”¢¢ŪČÜŅŗpH“óŠ”Ė³ŠņĪŖ£ØĢīŠņŗÅ£© > >  NH3”ü+CO32-+2H2O
NH3”ü+CO32-+2H2O NH3”ü+CO32-+2H2O£»£Ø4£©CH3COONH4ŹĒČõĖįČõ¼īŃĪ£¬ČÜŅŗ³ŹÖŠŠŌ£¬ĖµĆ÷CH3COO-ŗĶNH4+Ė®½ā³Ģ¶ČĻąĶ¬£¬µ«H2CO3ĖįŠŌČõÓŚCH3COOH£¬HCO3-µÄĖ®½ā³Ģ¶Č“óÓŚCH3COO-£¬ĖłŅŌNH4HCO3ČÜŅŗpH>7”£
NH3”ü+CO32-+2H2O£»£Ø4£©CH3COONH4ŹĒČõĖįČõ¼īŃĪ£¬ČÜŅŗ³ŹÖŠŠŌ£¬ĖµĆ÷CH3COO-ŗĶNH4+Ė®½ā³Ģ¶ČĻąĶ¬£¬µ«H2CO3ĖįŠŌČõÓŚCH3COOH£¬HCO3-µÄĖ®½ā³Ģ¶Č“óÓŚCH3COO-£¬ĖłŅŌNH4HCO3ČÜŅŗpH>7”£

 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®2 g H2Ėłŗ¬µÄŌ×ÓŹżÄæĪŖNA |
| B£®±ź×¼×“æöĻĀ£¬22.4 LĖ®ÖŠŗ¬ÓŠĖ®·Ö×ÓŹżĪŖNA |
| C£®³£ĪĀĻĀ£¬1 L 0.1 mol”¤LØD1µÄMgCl2ČÜŅŗÖŠŗ¬ÓŠµÄClØDŹżĪŖ0.2 NA |
| D£®³£ĪĀ³£Ń¹ĻĀ£¬11.2 L CH4ÖŠŗ¬ÓŠµÄĒāŌ×ÓŹżĪŖ2 NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®Cl-µÄ½į¹¹Ź¾ŅāĶ¼£ŗ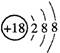 | B£®MgCl2µÄµē×ÓŹ½£ŗ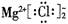 |
C£®CCl4µÄ½į¹¹Ź½£ŗ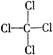 | D£®øßĀČĖįµÄ·Ö×ÓŹ½£ŗHClO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

A£®16gŃõĘųŗĶ³ōŃõµÄ»ģŗĻĪļÖŠŌ×ӵďżÄæ0.5NA | B£®1mol Cl2Óė×ćĮæµÄĢśĶźČ«·“Ó¦£¬×ŖŅʵĵē×ÓŹżĪŖ2NA | C£®1 L 0.5mol”¤L£1AlCl3ČÜŅŗÖŠŗ¬ÓŠµÄAl3+ŹżÄæĪŖ0.5NA | D£®ŌŚ±ź×¼×“æöĻĀ£¬22.4L HClÓė22.4L H2O2Ėłŗ¬ÓŠµÄµē×ÓŹż¾łĪŖ18 NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 MnO2+Zn+2H2SO4
MnO2+Zn+2H2SO4| A£®¢ŁÖŠMnO2ŗĶH2SO4¶¼ŹĒŃõ»Æ¼Į | B£®¢ŁÖŠÕŪ³ö16gSŹ±×ŖŅĘ1 molµē×Ó |
| C£®¢ŚÖŠMnSO4·¢ÉśŃõ»Æ·“Ó¦ | D£®ĮņĖįŌŚøĆÉś²śÖŠæÉŃ»·ĄūÓĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®»ÆŃ§Ä£ŠĶŹĒ¶ŌĪļÖŹ¼°Ęä±ä»ÆµÄ¼ņµ„»ÆÄ£Äā£¬ÓŠÖśÓŚ½āŹĶŅ»Š©»ÆѧĻÖĻó |
| B£®Ō×ÓŹĒ»Æѧ±ä»ÆÖŠµÄ×īŠ”Ī¢Į££¬Ķعżø“ŌӵĻÆѧ·“Ó¦æÉŅŌ²śÉśŠĀµÄŌŖĖŲ |
| C£®ÓŠµē×Ó×ŖŅĘµÄ¹ż³Ģ²»Ņ»¶ØÄܲśÉśµēĮ÷£¬µ«Ęä»Æѧ·“Ó¦Ņ»¶ØŹĒŃõ»Æ»¹Ō·“Ó¦ |
| D£®ČĪŗĪŗź¹ŪĪļÖŹ¶¼ŹĒÓÉĪ¢¹ŪĮ£×Ó¹¹³É£¬¶ųÕāŠ©Į£×ÓÖ®¼äÓÖ“ęŌŚ×ÅŅ»¶ØµÄ×÷ÓĆĮ¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĮņĖįĒā¼Ų¼ÓČČČŪČŚµÄµēĄė·½³ĢŹ½£ŗKHSO4 = K+ + H+ + SO42- |
| B£®ĀČ»Æļ§ČÜŅŗĻŌĖįŠŌµÄŌŅņ£ŗNH4++ H2O = NH3?H2O + H+ |
| C£®Ē¦Šīµē³Ų·ÅµēŹ±Ńō¼«·“Ó¦£ŗPb + SO42- - 2e-= PbSO4 |
| D£®ĀČ»ÆĢśČÜŅŗÖŠµĪ¼ÓÉŁĮæµÄĮņ»ÆÄĘČÜŅŗ£ŗ2Fe3+ + S2- = 2Fe2+ + S”ż |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com