
 2NH3(g) ��H����92.4kJ��mol��1����ش�
2NH3(g) ��H����92.4kJ��mol��1����ش� H2��NH3����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��
H2��NH3����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��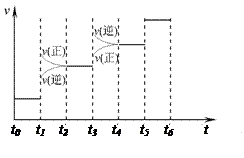
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2NH3��Ӧ�������б����У�����NH3���������ǣ�
2NH3��Ӧ�������б����У�����NH3���������ǣ�| A��3:1 | B��1:2 | C��2:1 | D��3:8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 H2��g��+I2��g��������ӦΪ���ȷ�Ӧ��
H2��g��+I2��g��������ӦΪ���ȷ�Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
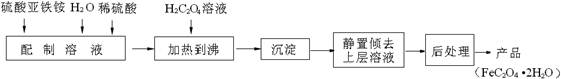
 6H2O��Һʱ���������ϡ���ᣬĿ����________________________________________________________��
6H2O��Һʱ���������ϡ���ᣬĿ����________________________________________________________��
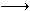 B������Ӧ�Ļ�ѧ����ʽΪ
B������Ӧ�Ļ�ѧ����ʽΪ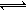 Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣�____________________________��
Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣�____________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 B + C��ijһ�¶�ʱ���ﵽƽ�⡣
B + C��ijһ�¶�ʱ���ﵽƽ�⡣ C��Ϊ���壬���������ƽ��___________�ƶ�(�������������)
C��Ϊ���壬���������ƽ��___________�ƶ�(�������������)�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
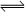 SO3(g)+NO(g) ,����һ���¶��£������ʵ���Ũ�Ⱦ�Ϊ2mol/L��SO2(g)��NO2(g)ע��һ�ܱ������У����ﵽƽ��״̬ʱ�����������SO2(g)��ת����Ϊ50%�������ڸ��¶��¡�
SO3(g)+NO(g) ,����һ���¶��£������ʵ���Ũ�Ⱦ�Ϊ2mol/L��SO2(g)��NO2(g)ע��һ�ܱ������У����ﵽƽ��״̬ʱ�����������SO2(g)��ת����Ϊ50%�������ڸ��¶��¡� �ⳣ����
�ⳣ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
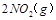
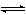
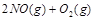 ������̶����ܱ���
������̶����ܱ��� ���н��У���Ӧ�ﵽƽ��״̬�ı�־��
���н��У���Ӧ�ﵽƽ��״̬�ı�־��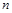
 ��ͬ������
��ͬ������
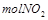
 ��������
��������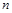
 ��ͬ������
��ͬ������
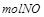
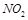 ��
��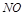 ��
�� �����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����֮��Ϊ2��2��1
�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����֮��Ϊ2��2��1| A���٢� | B���٢ۢ� | C���ڢ� | D������ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
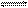 N2O4(��) ��H<0, �ﵽƽ���, ���ֱ�ı���������, ���´ﵽƽ��״̬��, �ܹ�ʹ��������ƽ����Է���������С���ǣ��� ��
N2O4(��) ��H<0, �ﵽƽ���, ���ֱ�ı���������, ���´ﵽƽ��״̬��, �ܹ�ʹ��������ƽ����Է���������С���ǣ��� ��| A��ͨ��N2 | B��ͨ��NO2 | C�������¶� | D��ͨ��N2O4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 H2O(g) + CO(g)��
H2O(g) + CO(g)��| A���ң��ף��� | B���ף������� | C���ң������� | D���ף��ң��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com