
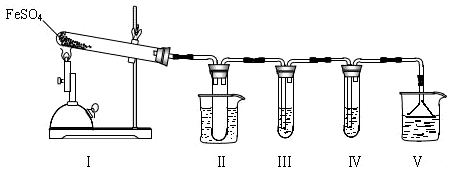
| �����Լ� | Ԥ������ͽ��� |
| װ�â���Թ��м���0.5 mol•L -1 BaCl2�� | ����������ɫ������֤����������к���SO3�� |
| װ�â����Թ��м���0.01 mol•L-1 ���� KMnO4 ��Һ����0.0l mol•L-1 ��ˮ���� | ����Һ��ɫ�����ɫ����ȥ��֤����������к���SO2������Һ��ɫ�����ɫ�������Ա仯��֤����������в���SO2 |

���� I��̽���ھƾ���Ƽ���������FeSO4�ֽ��������װ�â���Թ��в�װ�κ��Լ��Ƿ�ֹ��Һ������װ�â��У��Թܽ�����50���ˮԡ�У�Ŀ���Ƿ�ֹSO3Һ�������̣�ͨ��װ�â���Թ����Ȼ�����Һ֤�����ɲ�������������ͨ��װ�â����Թ������Ը��������Һ���ն�������֤����������Ĵ��ڣ����װ�â�������β����
��1�����ݰ�ȫƿ�������ǿ��Է�ֹ���������¶ȸ���44.8��CʱSO3Ϊ����״̬��
��2������FeSO4�ֽ������������ΪSO3��Ҳ������SO3��SO2�Ļ���SO3�������������ɰ�ɫ������SO2��ʹ������ػ���ˮ��ɫ��
��FeSO4������������Fe2��SO4��3����ȡ��Ʒ32.0g�������ϡ�����ܽ⣬��ˮ�ܽ⣬����250.00ml��Һ������ʽ�ζ�����ȡ25.00ml��Һ������������������������ӣ����������ˮ���������������������������ˡ�ϴ�ӡ����յõ���������
��1����ȡ��ȷ�Ƚϸߵ�������Һ�õ���ʽ�ζ��ܣ�
��2������Һ�еõ������������������ȷֽ�������������
��3������ԭ���غ���㣮
��� �⣺I����1�����в��������弫����Һ��Ӧʱ�����������������װ��II���Թ��ܷ�ֹ��Һ������װ�â��У���ȫƿ����SO3�ķе���44.8��C�����¶ȸ���44.8��CʱSO3Ϊ����״̬�����Թܽ�����50�����ˮԡ���ܷ�ֹSO3Һ�������̣�
�ʴ�Ϊ����ֹ��Һ������װ�â��У���ȫƿ������ֹSO3Һ�������̣�
��FeSO4�ֽ������������ΪSO3��Ҳ����ΪSO3��SO2�Ļ���SO3�������������ɰ�ɫ������SO2��ʹ������ػ���ˮ��ɫ��
�ʴ�Ϊ��
| 0.5 mol•L -1 BaCl2 | |
| 0.01 mol•L-1 ���� KMnO4 ��Һ����0.0l mol•L-1 ��ˮ�� | ����Һ��ɫ�����ɫ����ȥ��֤����������к���SO2������Һ��ɫ�����ɫ�������Ա仯��֤����������в���SO2 |
���� ������Ҫ�����˶�������Ͷ�������ļ��飬�����������ʵIJ����ǽ���Ĺؼ�����Ŀ�ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | PH=7 | B�� | c��H+��=c��OH-�� | ||
| C�� | c��H+��=c��OH-��=10-7mol/L | D�� | c��H+��•c��OH-��=10-14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����ȴ������ȶ� | B�� | ������������ڸ����� | ||
| C�� | H2S��HCl������ | D�� | ��̬HC1����̬H2S�ȶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��� | B�� | ������ | C�� | ̼���� | D�� | ͭ�ͽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
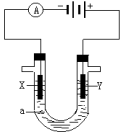 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ�������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ�������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶 A��B��D��C | B�� | ԭ������ D��C��B��A | ||
| C�� | ���Ӱ뾶 C��D��B��A | D�� | ���ʵĻ�ԭ�� A��B��D��C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ԣ�NH3��=0.04mol•L-1•min-1 | B�� | �ԣ�H2��=0.06mol•L-1•min-1 | ||
| C�� | �ԣ�N2��=0.06mol•L-1•min-1 | D�� | �ԣ�N2��=0.03mol•L-1•min-1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com