
��(Ti)����Ϊ��������֮��ĵ����������Ѱ�(TiO2)��Ŀǰ��õİ�ɫ���ϡ��Ʊ�TiO2��Ti��ԭ�����������ҹ�������������������λ������Fe2O3��������(��Ҫ�ɷ�ΪFeTiO3)��ȡTiO2���������£�
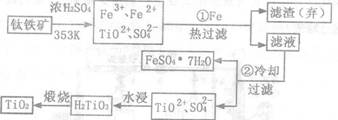
(1)����ټ�Fe��Ŀ�������������������������������������������������������� ��
�������ȴ��Ŀ����������������������������������������������������������������
(2)�����Ʊ�TiO2�Ĺ����У��������õĸ������������������� �����dzɱ��ͷ����ۺ��������أ���Һ��Ӧ���������������������������� ������
(3)�ɽ��ʯ(TiO2)��ȡ����Ti,�漰���IJ���Ϊ��TiO2��TiCl4����![]() �� Ti����ӦTiCl4+2Mg=2MgCl2+Ti��Ar�����н��е���������������������������������������
�� Ti����ӦTiCl4+2Mg=2MgCl2+Ti��Ar�����н��е���������������������������������������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)Ti��ԭ������Ϊ22,Tiλ��Ԫ�����ڱ��е�_________���ڣ���_________�塣
(2)����ټ�Fe��Ŀ����___________________________��
�������ȴ��Ŀ��________________________________��
(3)�����Ʊ�TiO2�Ĺ����У��������õĸ�������_________�����dzɱ��ͷ����ۺ��������أ���Һ��Ӧ����_________������
(4)�ɽ��ʯ(TiO2)��ȡ����Ti���漰���IJ���Ϊ��
TiO2![]() TiCl4
TiCl4![]() Ti
Ti
��֪����C(s)+O2(g)====CO2(g)����H=-393.5 kJ��mol-1
��2CO(g)+O2(g)====2CO2(g)����H=-566 kJ��mol-1
��TiO2(s)+2Cl2(g)====TiCl4(s)+O2(g)����H=+141 kJ��mol-1
��TiO2(s)+2Cl2(g)+2C(s)====TiCl4(s)+2CO(g)�Ħ�H=__________��
��ӦTiCl4+2Mg====2MgCl2+Ti��Ar�����н��е�������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��(Ti)����Ϊ��������֮��ĵ�������������ͼ��ʾ�����ѳ����ȼ�ͼ״�����ɲ�ҵ�����Դ�������Դ�����ʣ����ٻ�����Ⱦ������д���пհף�
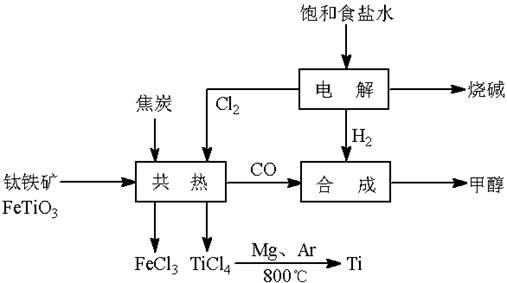
��1����ⱥ��ʳ��ˮʱ�������ĵ缫��ӦΪ ��
��2��д���������뽹̿��Cl2�����Ƶ����Ȼ��ѵĻ�ѧ����ʽ________________________��
��3����֪����Mg(s) + Cl2(g)��MgCl2(s)����H = �C 641 kJ/mol
![]() ��Ti(s) + 2Cl2(g)��TiCl4(s)����H = �C770 kJ/mol
��Ti(s) + 2Cl2(g)��TiCl4(s)����H = �C770 kJ/mol
��2Mg(s) + TiCl4(s)��2MgCl2(s) + Ti(s)����H�� ��
![]() ��Ӧ2Mg(s) + TiCl4(s)
��Ӧ2Mg(s) + TiCl4(s)![]() 2MgCl2(s) + Ti(s)����Ar�����н��е������� ��
2MgCl2(s) + Ti(s)����Ar������������ ��
��4����������ҵ���У��ϳ�96 t �״�����������H2 t (�������������������ʵ��κ���ʧ)��
��5���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء���֪��ȼ�ϵ�ص��ܷ�ӦʽΪ��2CH3OH + 3O2 + 4OH����2CO32�� + 6H2O����ȼ�ϵ�ط�����Ӧʱ��������Һ��pH (���������С�����䡱)���õ���и����ϵĵ缫��Ӧ��________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʮ��У�����ڶ���ģ��������ѧ�Ծ��������棩 ���ͣ������
��(Ti)����Ϊ��������֮��ĵ���������Ҳ����˵21�������ѵ����͡����ڵؿ��еĺ��������٣����ѵ�ұ��������δ���ͻ�ƣ�Ŀǰ��ֻ���ڼ������
����ͼ��ʾ�����ѳ����ȼ�ͼ״�����ɲ�ҵ���ɴ�������Դ�����ʣ����ٻ�����Ⱦ��

����д���пհף�
��1���ö��Ե缫���2 Lʳ��ˮʱ���ܷ�Ӧ�����ӷ���ʽ_______________________________���������ϲ���224 mL���壨��״����ʱ��������Һ��pH= ��������ǰ����Һ�������,ʳ��ˮ��������
��2��д���������������Ȼ��õ����Ȼ��ѵĻ�ѧ����ʽ ������ʾ��FeTiO3��TiΪ+4�ۣ�
��3����Ӧ2Mg��TiCl4 2MgCl4��Ti��Ar�����н��е�������____________________��
2MgCl4��Ti��Ar������������____________________��
��4����������һ����Ҫ�����ȼ�ϣ�����ͨ���״����Ӽ���ˮ�Ƶã�
2CH3OH(g) CH3OCH3(g)+H2O(g)����H=" -23.5" kJ/mol
CH3OCH3(g)+H2O(g)����H=" -23.5" kJ/mol
T1 ��ʱ���ں����ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯����ͼ��ʾ��

��T1 ��ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ������������
����ͬ�����£����ı���ʼŨ�ȣ�ijʱ�̸����Ũ������Ϊc(CH3OH)="0.4" mol/L��c(H2O)="0.6" mol/L��(CH3OCH3)="1.2" mol/L����ʱ�����淴Ӧ���ʵĴ�С��v��������v��(�>������<����=��)��
��5����������ҵ���У��ϳ�192�ּ״�����������ⲹ��H2__________�� ���������������������ʵ��κ���ʧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʦ����2010�����������¿������ۣ����⻯ѧ���� ���ͣ������
(13��) ��(Ti)����Ϊ��������֮��ĵ�������������ͼ��ʾ�����ѳ����ȼ�ͼ״�����ɲ�ҵ�����Դ�������Դ�����ʣ����ٻ�����Ⱦ������д���пհף�
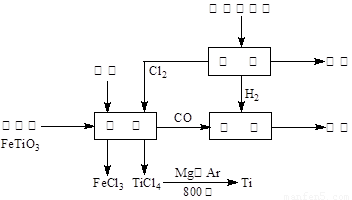
��1����ⱥ��ʳ��ˮʱ�������ĵ缫��ӦΪ ��
��2��д���������뽹̿��Cl2�����Ƶ����Ȼ��ѵĻ�ѧ����ʽ________________________��
��3����֪����Mg(s) + Cl2(g)��MgCl2(s)����H = �C 641 kJ/mol
��Ti(s) + 2Cl2(g)��TiCl4(s)����H = �C770 kJ/mol
��2Mg(s) + TiCl4(s)��2MgCl2(s) + Ti(s)����H�� ��
��Ӧ2Mg(s) + TiCl4(s) 2MgCl2(s) + Ti(s)����Ar�����н��е�������
��
2MgCl2(s) + Ti(s)����Ar������������
��
��4����������ҵ���У��ϳ�96 t �״�����������H2 t (�������������������ʵ��κ���ʧ)��
��5���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء���֪��ȼ�ϵ�ص��ܷ�ӦʽΪ��2CH3OH + 3O2 + 4OH����2CO32�� + 6H2O����ȼ�ϵ�ط�����Ӧʱ��������Һ��pH (���������С�����䡱)���õ���и����ϵĵ缫��Ӧ��________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡ��ɽ�и�����ѧģ���Ծ����ߣ� ���ͣ������
��(Ti )����Ϊ��������֮��ĵ�������������ͼ��ʾ�����ѳ����ȼ�ͼ״�����ɲ�ҵ�����Դ�������Դ�����ʣ����ٻ�����Ⱦ������д���пհף�


��l����ⱥ��ʳ��ˮʱ���ܷ�Ӧ�����ӷ���ʽ�� ��
 ��2��д���������Ȼ����õ����Ȼ��ѵĻ�ѧ����
��
��2��д���������Ȼ����õ����Ȼ��ѵĻ�ѧ����
��
��3����֪����Mg(s) + Cl2(g)��MgCl2(s) ��H =��641kJ��mol-1
 ��Ti(s)
+ 2Cl2(g)��TiCl4(s) ��H
= ��770kJ��mol-1
��Ti(s)
+ 2Cl2(g)��TiCl4(s) ��H
= ��770kJ��mol-1
��2Mg(s) + TiCl4(s)��2MgCl2(s) + Ti(s) ��H�� ��
 ��Ӧ2Mg(s)
+ TiCl4(s)
��Ӧ2Mg(s)
+ TiCl4(s) 2MgCl2(s)
+ Ti����Ar�����н��е������ǣ�
______________________________________
��
2MgCl2(s)
+ Ti����Ar�����н��е������ǣ�
______________________________________
��
 ��4���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ���и����ϵĵ缫��Ӧʽ��
��
��4���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ���и����ϵĵ缫��Ӧʽ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com