
 ��A��B��C��D��E��F���ֶ�����Ԫ�أ���Ԫ��������Ϣ���±���
��A��B��C��D��E��F���ֶ�����Ԫ�أ���Ԫ��������Ϣ���±���| Ԫ�ر�� | Ԫ��������Ϣ |
| A | A�ĵ������ܶ���С������ |
| B | B�ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ������������ |
| C | C��ԭ�����������������ڲ������������ |
| D | D��Bͬ���ڣ���������D�ļ����Ӱ뾶��С |
| E | B��C��E��ɵ�36���ӵĻ�����Y�Ǽ�������������Ҫ�ɷ� |
| F | FԪ���������������۵Ĵ�����Ϊ4 |
���� A�ĵ������ܶ���С�����ʣ���A����Ԫ�أ�
B�ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ�����������ӣ���B�Ƕ�����Ԫ�أ�����B����Ԫ�أ�
C��ԭ�����������������ڲ����������������C�Ƕ�����Ԫ�أ�����C����Ԫ�أ�
D��Bͬ���ڣ���������D�ļ����Ӱ뾶��С����D����Ԫ�أ�
B��C��E��ɵ�36���ӵĻ�����Y�Ǽ�������������Ҫ�ɷ֣�����������Ҫ�ɷ��Ǵ������ƣ�����E����Ԫ�أ�
FԪ���������������۵Ĵ�����Ϊ4����F�Ƕ�����Ԫ�أ�����F����Ԫ�أ��ݴ˽��н��
��� �⣺��1�������ӵĵ��Ӳ���С�������ӡ������ӵĵ��Ӳ��������Ӳ���Խ�࣬���Ӱ뾶Խ�����������Ӱ뾶С�������Ӻ������Ӱ뾶�����Ӳ�����ͬ�����ӣ����Ӱ뾶����ԭ���������������С�����������Ӱ뾶���������Ӱ뾶�������Ӱ뾶��СΪ��S2-��Cl-��Al3+��
�ʴ�Ϊ��S2-��Cl-��Al3+��
��2��D��EԪ���γɵĻ�����Ϊ�Ȼ����������Ӳ���ˮ�⣬ˮ��Һ�����ԣ�����Һ������Ũ�ȵĴ�С˳��Ϊ��c��Cl-����c��Al3+����c��H+����c��OH-����
�ʴ�Ϊ��c��Cl-����c��Al3+����c��H+����c��OH-����
��3������������Һ��ǿ���ԣ�������������������ʽ�Σ������������ƺ����������Ʒ�Ӧ���������ơ�ˮ�Ͷ�������Ӧ�����ӷ���ʽΪ��H++HSO-3=SO2��+H2O��
�ʴ�Ϊ��H++HSO-3=SO2��+H2O��
��4���������Ļ�����������ϡ�����������������������������������������������Һ�м����������������Һ����������������������ƫ��������Һ��������ϴ�ӡ�������պ�õ�һ�ֹ����������������������������������Ļ����������ȣ��������������൱����Ԫ�ص�����������������������=$\frac{16��3}{16��3+56��2}$��100%=30%��
�ʴ�Ϊ��30%��
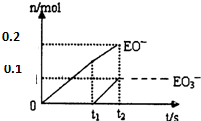
��5������ͼ��֪������������ӵ����ʵ���Ϊ0.2mol����������ӵ����ʵ���Ϊ0.1mol�����Դ���������ӵ����ʵ�������������ӵ����ʵ���֮��Ϊ2��1�����ݵ�ʧ�����غ�֪���������������Ƶķ�Ӧ����ʽΪ��10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O��
����Ҫ�������Ƶ�����Ϊx��
10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O
740g 2mol
x 0.1mol
��ã�x=37g��
�ʴ�Ϊ��37��10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O��
��6���⻯�����Ҵ���Ӧ�����Ҵ��ƺ���������Ӧ�Ļ�ѧ����ʽΪ��NaH+CH3CH2OH=CH3CH2ONa+H2����
�ʴ�Ϊ��NaH+CH3CH2OH=CH3CH2ONa+H2����
���� ���⿼��λ�ýṹ���ʵ����ϵ��Ӧ�ã���Ŀ�Ѷ��еȣ��漰������ԭ��Ӧ�ļ��㡢���ӷ���ʽ����д��Ԫ�������ɵ�֪ʶ�㣬�ۺ��Խ�ǿ���ѵ��ǣ�5������йؼ��㣬����ԭ���غ㼰ת�Ƶ����غ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
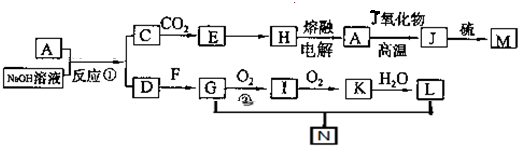
 ��M�Ļ�ѧʽΪFeS��F�ĵ���ʽΪ
��M�Ļ�ѧʽΪFeS��F�ĵ���ʽΪ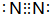 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C2H4��C2H2 | B�� | CH4��C2H6 | C�� | CH4��C2H4 | D�� | C2H2��C3H6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 10 mL 0.1 mol/L��ˮ��10 mL 0.1 mol/L�����ϣ�c��Cl-����c�� NH4+����c��OH-����c��H+�� | |
| B�� | 10 mL 0.1 mol/L NH4Cl��Һ��5 mL 0.2 mol/L NaOH��Һ��ϣ�c��Na+��=c��Cl-����c��OH-����c��H+�� | |
| C�� | 10 mL 0.1 mol/L CH3COOH��Һ��5 mL 0.2 mol/L NaOH��Һ��ϣ�c��Na+��=c��CH3COO-����c��OH-����c��H+�� | |
| D�� | 10 mL 0.5 mol/L CH3COONa��Һ��6 mL 1 mol/L�����ϣ�c��Cl-����c��Na+����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H+��K+��SO42-��Cl- | B�� | OH-��Na+��Cu2+��SO42- | ||
| C�� | ClO-��K+��Na+��Cl- | D�� | Ag+��NO3-��Br-��NH4+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
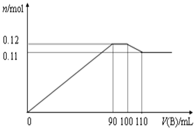 ��100mLBaCl2��AlCl3��FeCl3 �Ļ����ҺA����μ���Na2SO4��NaOH�Ļ����ҺB���������������ʵ���n�ͼ�����ҺB�������ϵ��ͼ��ʾ��
��100mLBaCl2��AlCl3��FeCl3 �Ļ����ҺA����μ���Na2SO4��NaOH�Ļ����ҺB���������������ʵ���n�ͼ�����ҺB�������ϵ��ͼ��ʾ��| ���� | Na2SO4 | NaOH | BaCl2 | AlCl3 | FeCl3 |
| c/mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ���� | V���ռ���Һ��/mL | V��HCl��/mL | |
| ������ | ĩ���� | ||
| 1 | 20.00 | 0.00 | 31.00 |
| 2 | 20.00 | 1.00 | 32.04 |
| 3 | 20.00 | 1.10 | 32.18 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com