
| 50mLŃĪĖį | 50mLŃĪĖį | 50mLŃĪĖį | |
| m£Ø»ģŗĻĪļ£© | 9.2g | 15.7g | 27.6g |
| V£ØCO2£©£Ø±źæö£© | 2.24L | 3.36L | 3.36L |
| n |
| V |
| 3.36L |
| 22.4L/mol |
| 0.15mol |
| 0.05L |
| 2.24L |
| 22.4L/mol |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
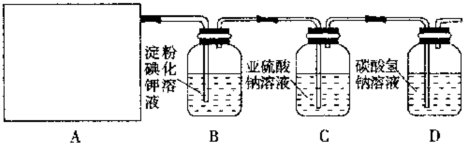
£Ø12·Ö£© Ä³ŃŠ¾æŠŌѧĻ°Š”×éĶ¬Ń§Éč¼ĘĮĖČēĻĀĶ¼ÖĘČ”ĀČĘų²¢ŃéÖ¤ĘäŠŌÖŹµÄŹµŃé×°ÖĆ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©øĆŹµŃéÖŠA²æ·ÖµÄ×°ÖĆŹĒ £ØĢīŠ“×°ÖƵĊņŗÅ£©
£Ø2£©AÖŠ·¢Éś·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬BÖŠµÄĻÖĻóŹĒ £»
ÕūĢ׏µŃé×°ÖĆ“ęŌŚµÄĆ÷ĻŌȱĻŻŹĒ ”£
£Ø3£©Š“³öD×°ÖĆÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
£Ø4£©Š“³öC×°ÖĆÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£ĒėÄć°ļøĆŠ”×éĶ¬Ń§Éč¼ĘŅ»øöŹµŃ飬֤Ć÷Ļ“ĘųĘæCÖŠµÄ![]() Ņѱ»Ńõ»Æ£Ø¼ņŹöŹµŃé²½Öč£©£ŗ
Ņѱ»Ńõ»Æ£Ø¼ņŹöŹµŃé²½Öč£©£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ä³ŃŠ¾æŠŌѧĻ°Š”×éĶ¬Ń§Éč¼ĘĮĖČēĻĀĶ¼ÖĘČ”ĀČĘų²¢ŃéÖ¤ĘäŠŌÖŹµÄŹµŃé×°ÖĆ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©øĆŹµŃéÖŠA²æ·ÖµÄ×°ÖĆŹĒ £ØĢīŠ“×°ÖƵĊņŗÅ£©
£Ø2£©AÖŠ·¢Éś·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬BÖŠµÄĻÖĻóŹĒ £»
ÕūĢ׏µŃé×°ÖĆ“ęŌŚµÄĆ÷ĻŌȱĻŻŹĒ ”£
£Ø3£©Š“³öD×°ÖĆÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
£Ø4£©ĒėÄć°ļøĆŠ”×éĶ¬Ń§Éč¼ĘŅ»øöŹµŃ飬֤Ć÷Ļ“ĘųĘæCÖŠµÄ![]() Ņѱ»Ńõ»Æ£Ø¼ņŹöŹµŃé²½Öč£©£ŗ ”£
Ņѱ»Ńõ»Æ£Ø¼ņŹöŹµŃé²½Öč£©£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğø£½ØŹ”øßČżÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
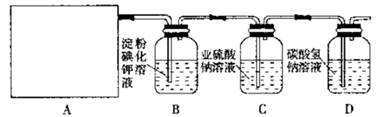
Ä³ŃŠ¾æŠŌѧĻ°Š”×éĶ¬Ń§Éč¼ĘĮĖČēĻĀĶ¼ÖĘČ”ĀČĘų²¢ŃéÖ¤ĘäŠŌÖŹµÄŹµŃé×°ÖĆ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©øĆŹµŃéÖŠA²æ·ÖµÄ×°ÖĆŹĒ £ØĢīŠ“×°ÖƵĊņŗÅ£©
£Ø2£©AÖŠ·¢Éś·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬BÖŠµÄĻÖĻóŹĒ £»
ÕūĢ׏µŃé×°ÖĆ“ęŌŚµÄĆ÷ĻŌȱĻŻŹĒ ”£
£Ø3£©Š“³öD×°ÖĆÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
£Ø4£©ĒėÄć°ļøĆŠ”×éĶ¬Ń§Éč¼ĘŅ»øöŹµŃ飬֤Ć÷Ļ“ĘųĘæCÖŠµÄ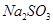 Ņѱ»Ńõ»Æ£Ø¼ņŹöŹµŃé²½Öč£©£ŗ
ӣ
Ņѱ»Ńõ»Æ£Ø¼ņŹöŹµŃé²½Öč£©£ŗ
ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com