
ЁОЬтФПЁПФГЭЌбЇгћХфжЦЗћКЯЯТСаЬѕМўЕФШмвКЃЌЦфжаПЩФмЪЕЯжЕФЪЧ
A. жЛКЌ0.1 mol Na+ЁЂ0.2mol Mg2ЃЋЁЂ0.1 mol Cl-КЭ0.1 mol NO3-ЕФШмвК
B. жЛКЌ0.1mol NH4+ЁЂ0.1 mol Ca2ЃЋЁЂ0.1 mol CO32-КЭ0.1mol Cl-ЕФШмвК
C. дкБъзМзДПіЯТЃЌНЋVLЦјЬхЃЈФІЖћжЪСПЪЧM g/molЃЉШмгк0.1LЫЎжаЃЌЫљЕУШмвКЕФУмЖШЮЊd g/mLЃЌдђДЫШмвКЕФЮяжЪЕФСПХЈЖШЮЊ1000Vd/ЃЈ2240+VMЃЉmol/L
D. ПЩгУ1000mLШнСПЦПЁЂЩеБЁЂВЃСЇАєЁЂСПЭВЁЂ58.5gNaClЙЬЬхКЭЫЎХфжЦ1L1mol/LЕФNaClШмвК
ЁОД№АИЁПC
ЁОНтЮіЁП
AЁЂШмвКвЊГЪЕчжаад,МДбєРызгЫљДјЕФе§ЕчКЩЪ§вЊгывѕРызгЫљДјЕФИКЕчКЩЪ§ЯрЕШ,ЖјжЛКЌ0.1 mol Na+ЁЂ0.2mol Mg2ЃЋЁЂ0.1 mol Cl-КЭ0.1 mol NO3-ЕФШмвКжа,бєРызгЫљДјЕФе§ЕчКЩЕФЮяжЪЕФСПn=0.1mol+0.2molЁС2=0.5mol,вѕРызгЫљДјЕФИКЕчКЩЕФЮяжЪЕФСПn=0.1mol+0.1mol=0.2mol,ШмвКВЛГЪЕчжаадЃЌЙЪAДэЮѓ;
BЁЂдкШмвКжа, Ca2ЃЋКЭ CO32-ЗДгІЩњГЩЬМЫсИЦГСЕэЃЌЫљвдCa2ЃЋКЭ CO32-ВЛФмЙВДцЃЌЙЪBДэЮѓ;
CЁЂдкБъзМзДПіЯТЃЌНЋVLЦјЬхЃЈФІЖћжЪСПЪЧM g/molЃЉШмгк0.1LЫЎжаЃЌШмжЪЕФЮяжЪЕФСПЮЊVLЁТ22.4L/mol=V/22.4molЃЌШмвКЕФЬхЛ§ЮЊV=m/![]() =(V/22.4molЁСM g/mol+100g)/d g/mLЃЌЫљвдДЫШмвКЕФЮяжЪЕФСПХЈЖШЮЊV/22.4molЁТ[1000Vd/(V/22.4molЁСM g/mol+100g)/d g/mL]=ЃЈ2240+VMЃЉmol/L,ЙЪCе§ШЗЃЛ
=(V/22.4molЁСM g/mol+100g)/d g/mLЃЌЫљвдДЫШмвКЕФЮяжЪЕФСПХЈЖШЮЊV/22.4molЁТ[1000Vd/(V/22.4molЁСM g/mol+100g)/d g/mL]=ЃЈ2240+VMЃЉmol/L,ЙЪCе§ШЗЃЛ
DЁЂХфжЦ1L1mol/LЕФNaClШмвКдкЖЈШнЪБашвЊгУЕННКЭЗЕЮЙм,ГЦСПЙЬЬхЪБашвЊгУЕНЬьЦНЃЌЙЪDДэЮѓ.
злЩЯЫљЪіЃЌБОЬтгІбЁCЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгУNAБэЪОАЂЗќМгЕТТоГЃЪ§ЕФжЕЃЌЯТСаа№Ъіе§ШЗЕФЪЧ(ЁЁЁЁ)
A. 1 molыВЦјдкБъзМзДПіЯТЕФЬхЛ§дМЮЊ11.2 L
B. 20 ЁцЁЂ10 MPaзДЬЌЯТЃЌ32 g O2КЭO3ЕФЛьКЯЦјЬхЫљКЌдзгЪ§ЮЊ2.5NA
C. БъзМзДПіЯТЃЌ11.2 L H2CO3КЌгаЕФдзгЪ§ЮЊ3NA
D. ГЃЮТГЃбЙЯТЃЌ44 g CO2КЌгаЕФдзгЪ§ЮЊ3NA
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПН№ЪєМАЦфЛЏКЯЮядкПЦбЇбаОПКЭЙЄвЕЩњВњжаОпгаживЊЕФгУЭОЁЃ
ЃЈ1ЃЉШ§ТШЛЏЬњШмвКгУгкМьбщЪГгУЯуОЋввѕЃввЫсввѕЅЪБЃЌЛсЩњГЩзЯЩЋХфКЯЮяЃЌЦфХфРызгНсЙЙШчЭМЫљЪОЃК
ЂйДЫХфКЯЮяжаЃЌЬњРызгМлЕчзгХХВМЭМЮЊ_____ЃЛ
ЂкДЫХфРызгжаЬМдзгЕФдгЛЏЙьЕРРраЭЮЊ_____ЃЛ
ЂлДЫХфРызгжаКЌгаЕФЛЏбЇМќЮЊ_____ЁЃ
AЃЎРызгМќ BЃЎН№ЪєМќ CЃЎМЋадМќ DЃЎЗЧМЋадМќ EЃЎХфЮЛМќ FЃЎЧтМќ GЃЎІвМќ HЃЎІаМќ
ЃЈ2ЃЉNO2-гыюмбЮаЮГЩЕФХфРызг[Co(NO2)6]3-ПЩгУгкМьбщK+ЕФДцдкЁЃNO2-РызгЕФVSEPRФЃаЭУћГЦЮЊ_____ЃЌK3[Co(NO2)6]ЪЧЛЦЩЋГСЕэЃЌИУЮяжЪжаЫФжждЊЫиЕФЕчИКадгЩДѓЕНаЁЕФЫГађЪЧ_____ЁЃ
ЃЈ3ЃЉбаОПЮяжЪДХадБэУїЃКН№ЪєбєРызгКЌЮДГЩЖдЕчзгдНЖрЃЌдђДХаддНДѓЃЌДХМЧТМадФмдНКУЁЃРызгаЭбѕЛЏЮяV2O5КЭCrO2ЃЌЦфжаЪЪКЯзїТМвєДјДХЗлдСЯЕФЪЧ_____ЁЃ

ЃЈ4ЃЉУЬЕФвЛжжХфКЯЮяЕФЛЏбЇЪНЮЊMn(BH4)2(THF)3ЃЌаДГіСНжжгыBH4-ЛЅЮЊЕШЕчзгЬхЕФЮЂСЃ_____ЃЈЧыаДвЛИіЗжзгКЭвЛИіРызгЃЉЁЃ
ЃЈ5ЃЉZnSдкгЋЙтЬхЁЂЙтЕМЬхВФСЯЁЂЭПСЯЁЂбеСЯЕШаавЕжагІгУЙуЗКЃЌСЂЗНZnSОЇЬхЕФНсЙЙШчЭМЫљЪОЃЌaЕФХфЮЛЪ§ЮЊ_____ЃЌвбжЊОЇАћУмЖШЮЊІб g/cm3ЃЌдђЯрСк2ИіbЮЂСЃжЎМфЕФОрРыЮЊ_____nmЃЈСаМЦЫуЪНЃЉЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПIЁЂЮЊВтЖЈН№ЪєФЦбљЦЗЃЈБэУцга Na2OЃЉжаФЦЕЅжЪЕФжЪСПЗжЪ§ЃЌЩшМЦСЫШчЯТЪЕбщЃЈЗДгІзАжУШчгвЭМ ЫљЪОЃЉЃК
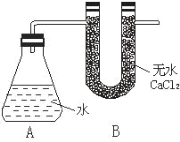
ЂйГЦСП AЁЂB ЕФзмжЪСП
ЂкГЦШЁвЛЖЈжЪСПЕФФЦбљЦЗ
ЂлНЋФЦбљЦЗЭЖШызЖаЮЦПжаЃЌбИЫйШћНєДј U аЮИЩдяЙмЃЈФкКЌЮоЫЎ CaCl2 ИЩдя МСЃЉЕФЯ№ЦЄШћ гаЙиЪ§ОнЪЧЃКГЦШЁЕФН№ЪєФЦбљЦЗжЪСПЮЊ a gЃЌAЁЂB ЗДгІЧАзмжЪСПЮЊ b gЃЌЗДгІКѓ AЁЂB ЕФзмжЪСПЮЊ c gЁЃ ЧыИљОнЬтвтЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉA жаФЦКЭЫЎЗДгІЙ§ГЬПЩПДЕНФЦШлГЩЩСССЕФаЁЧђЃЌВњЩњетвЛЯжЯѓдвђЪЧЃК_____________
ЃЈ2ЃЉгУ aЁЂbЁЂc БэЪОЕФФЦЕЅжЪЕФжЪСПЗжЪ§ЮЊ_______________
ЃЈ3ЃЉШчЙћУЛга B зАжУЖдЪЕбщНсЙћгаКЮгАЯь___________ЁЃЃЈЬюЁАЦЋДѓЁБЛђЁАЦЋаЁЁБЁАВЛгАЯьЁБЃЉ
IIЁЂЯжгУН№ЪєФЦКЭПеЦјжЦБИДПЖШНЯИпЕФ Na2O2ЃЌПЩРћгУЕФзАжУШчЯТЭМЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЈЬсЪОЃКNa2O2 ПЩвдгы H2OЁЂCO2 ЗДгІЃЉ
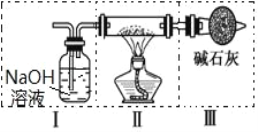
ЃЈ4ЃЉЩЯЪізАжУЂєжаЪЂЗХЕФЪдМСЪЧ_____ЃЌЮЊЭъГЩЪЕбщгІНЋзАжУЂєНгдк_____ЃЈЬюаДзжФИКХЃЉЁЃ
A.I жЎЧА B. I КЭ II жЎМф C. II КЭ III жЎМф D. III жЎКѓ
ЃЈ5ЃЉЕуШМОЦОЋЕЦКѓЃЌЙлВьЕНзАжУ II жаЕФЯжЯѓЮЊ_____ЁЃ
ЃЈ6ЃЉзАжУ II жа Na ЩњГЩ Na2O2 ЕФЛЏбЇЗДгІЗНГЬЪНЮЊ_____ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПютЫсФЦОЇЬхЃЈNa2MoO4ЁЄ2H2OЃЉГЃгУгкжЦдьзшШММСКЭЮоЙЋКІаЭРфЫЎЯЕЭГЕФН№ЪєвжжЦМСЁЃЯТЭМЪЧРћгУютОЋПѓЃЈжївЊГЩЗжЪЧMoS2ЃЌКЌЩйСПPbSЕШЃЉЮЊдСЯЩњВњютЫсФЦОЇЬхЕФЙЄвеСїГЬЭМЃК

ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЬсИпБКЩеаЇТЪЕФЗНЗЈга____________ЁЃЃЈаДвЛжжЃЉ
ЃЈ2ЃЉЁАБКЩеЁБЪБMoS2зЊЛЏЮЊMoO3ЃЌИУЗДгІЙ§ГЬЕФЛЏбЇЗНГЬЪНЮЊ________________________ЃЌбѕЛЏВњЮяЪЧ________ЃЈаДЛЏбЇЪНЃЉЁЃ
ЃЈ3ЃЉЁАМюНўЁБЪБКЌютЛЏКЯЮяЗЂЩњЕФжївЊЗДгІЕФЛЏбЇЗНГЬЪНЮЊ__________________________ЁЃ
ЃЈ4ЃЉШєЁАГ§жиН№ЪєРызгЁБЪБМгШыЕФГСЕэМСЮЊNa2SЃЌдђЗЯдќГЩЗжЕФЛЏбЇЪНЮЊ________ЁЃ
ЃЈ5ЃЉВтЕУЁАГ§жиН№ЪєРызгЁБжаВПЗжРызгЕФХЈЖШЃКc(MoO42-)=0.40mol/LЃЌc(SO42-)=0.04mol/LЁЃЁАНсОЇЁБЧАашЯШГ§ШЅSO42-ЃЌЗНЗЈЪЧМгШыBa(OH)2ЙЬЬхЁЃМйЩшМгШыBa(OH)2ЙЬЬхКѓШмвКЬхЛ§ВЛБфЃЌЕБSO42-ЭъШЋГСЕэЃЈc(SO42-)Ём1.0ЁС10-5mol/LЃЉЪБЃЌBaMoO4ЪЧЗёЛсЮіГіЃП____________________________________ЁЃЃЈЧыМЦЫуЫЕУїЃЉ[вбжЊЃКKsp(BaSO4)=1.1ЁС10-10ЃЌKsp(BaMoO4)=4.0ЁС10-8]
ЃЈ6ЃЉютОЋПѓдкМюадЬѕМўЯТЃЌМгШыNaClOШмвКЃЌвВПЩвджЦБИютЫсФЦЃЌЭЌЪБгаSO42-ЩњГЩЃЌИУЗДгІЕФРызгЗНГЬЪНЮЊ___________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЛЏКЯЮяG[ ]ЪЧвЛжжвНвЉжаМфЬхЃЌЫќЕФвЛжжКЯГЩТЗЯпШчЯТЃК
]ЪЧвЛжжвНвЉжаМфЬхЃЌЫќЕФвЛжжКЯГЩТЗЯпШчЯТЃК

вбжЊЃК
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉAЕФУћГЦЪЧ_____________ЁЃ
ЃЈ2ЃЉBЁњCЕФЗДгІЬѕМўЮЊ_____________ЁЃ
AЁњBКЭDЁњEЕФЗДгІРраЭЗжБ№ЪЧ_____________ЁЂ_____________ЁЃ
ЃЈ3ЃЉDдкХЈСђЫсМгШШЕФЬѕМўЯТЛсЩњГЩвЛжжКЌСљдЊЛЗЕФЛЏКЯЮяЃЌИУЛЏКЯЮяЕФНсЙЙМђЪНЮЊ_____________ЁЃЃЈ4ЃЉHЪЧвЛжжИпОлѕЅЃЌDЁњHЕФЛЏбЇЗНГЬЪНЮЊ_____________ЁЃ
ЃЈ5ЃЉЯТСаЙигкЛЏКЯЮяGЕФЫЕЗЈДэЮѓЕФЪЧ_____________ЁЃ
A.GЕФЗжзгЪНЮЊC12H14O5
B.1molGгыNaOHШмвКМгШШзюЖрЯћКФ2molNaOH
C.вЛЖЈЬѕМўЯТGЗЂЩњЯћШЅЗДгІЩњГЩЕФгаЛњЮяДцдкЫГЗДвьЙЙЬх
D.дквЛЖЈЬѕМўЯТGФмгыHBrЗЂЩњШЁДњЗДгІ
ЃЈ6ЃЉMЪЧDЕФЭЌЗжвьЙЙЬхЃЌгыDОпгаЯрЭЌЕФЙйФмЭХЁЃдђMПЩФмЕФНсЙЙга____жжЁЃ
ЃЈ7ЃЉвбжЊЗгєЧЛљВЛвзгыєШЫсЗЂЩњѕЅЛЏЗДгІЃЌаДГігЩБНЗгЃЌМзБНЮЊдСЯжЦБИБНМзЫсБНЗгѕЅЃЈ![]() ЃЉЕФКЯГЩТЗЯп(ЦфЫќЪдМСШЮбЁ)ЁЃ __________________
ЃЉЕФКЯГЩТЗЯп(ЦфЫќЪдМСШЮбЁ)ЁЃ __________________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаХХСаЫГађе§ШЗЕФЪЧЃЈ ЃЉ
A.ШШЮШЖЈадЃКH2OЃОHFЃОH2SB.ЗЧН№ЪєадЃКClЃОSЃОSi
C.зюИпе§ЛЏКЯМлЃКFЃОNЃОCD.ЫсадЃКH2CO3ЃОH3PO4ЃОH2SO4
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊAЪЧвЛжжЪГЦЗжаГЃМћЕФгаЛњЮяЃЌдкЪЕбщЪвжаЃЌЦјЬхBФмЪЙфхЫЎЭЪЩЋЩњГЩCЃЌЮяжЪFЕФЗжзгЪНЮЊC4H8O2ЃЌЪЧвЛжжгаХЈгєЯуЮЖЃЌВЛвзШмгкЫЎЕФгЭзДвКЬхЃЌЧыИљОнвдЯТаХЯЂЛиД№
ЯрЙиЮЪЬтЃК

ЧыЛиД№ЃК
ЃЈ1ЃЉЮяжЪEжаЕФЙйФмЭХУћГЦЮЊЃК____________ЁЃ
ЃЈ2ЃЉЮяжЪBгыфхЫЎЗДгІЩњГЩCЕФЗДгІРраЭЮЊЃК
____________________________________________________________________ЁЃ
BгыEдквЛЖЈЬѕМўЯТЗДгІЩњГЩFЕФЛЏбЇЗНГЬЪНЮЊЃК
____________________________________________________________________ЁЃ
ЃЈ4ЃЉXЪЧгаЛњЮяEЕФЭЌЯЕЮяЃЌЦфЯрЖдЗжзгжЪСПЪЧ74ЃЌЧвЛЏбЇаджЪгыEЯрЫЦЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ_____ЃЉ
A.гаЛњЮяEФмЗЂЩњвјОЕЗДгІ B.гаЛњЮяAгыXЗДгІЩњГЩБћЫсМзѕЅ
C.МзЫсМзѕЅЪЧXЕФЭЌЗжвьЙЙЬх D.гаЛњЮяAЁЂDЁЂEОљФмШмНтгкЫЎ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯжга14.4gCOКЭCO2ЕФЛьКЯЦјЬх,дкБъзМзДПіЯТЦфЬхЛ§ЮЊ8.96L.
ЧыЛиД№ЯТСаЮЪЬт:
ЃЈ1ЃЉИУЛьКЯЦјЬхЕФЦНОљФІЖћжЪСПЮЊ_____.
ЃЈ2ЃЉЛьКЯЦјЬхжаЬМдзгЕФИіЪ§ЮЊ_____(гУNAБэЪОАЂЗќМгЕТТоГЃЪ§ЕФжЕ).
ЃЈ3ЃЉНЋЛьКЯЦјЬхвРДЮЭЈЙ§ШчЭМЫљЪОзАжУ,зюКѓЪеМЏдкЦјЧђжа(ЬхЛ§дкБъзМзДПіЯТВтЖЈ).
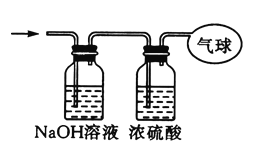
ЂйЦјЧђЪеМЏЕНЕФЦјЬхЕФФІЖћжЪСПЮЊ_____.
ЂкЦјЧђЪеМЏЕНЕФЦјЬх,ЕчзгзмЪ§ЮЊ_____(гУNAБэЪОАЂЗќМгЕТТоГЃЪ§ЕФжЕ).
ЂлЦјЧђЕФЬхЛ§ЮЊ_____L.
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com