
��12�֣�ijѧ��ʵ����ȤС������ͼ1װ������ɡ�NaHCO3��NaCl�������NaHCO3�����IJⶨ����ʵ�顣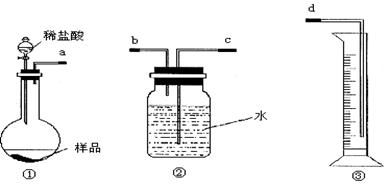
ͼ1
��1���������ӿ�����˳����_________________���ýӿ���ĸ��д����
��2����װ�����Ӻö�δװҩƷǰ�����������ԵIJ�����_________________________��
��3����ͬѧ��Ϊ�����������ϴ�������¸Ľ���ʩ������Ϊ���е���______��������ţ�
| A���ڵ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2���� |
| B����װ�â���ϡ���ỻ��ϡ���ᣬװ�â���ˮ���ɱ���NaHCO3��Һ |
| C����װ�â���ˮ���ɱ���Na2CO3��Һ |
| D���μ�����˹��� |
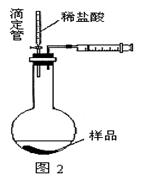

��1��a��b��c��d��2�֣�
��2����1���ڢ��м�ˮ��û�����ܣ��رշ�Һ©��������������סԲ����ƿ����ƾ����ȣ��������г�������Һ����������˵�������Ժã���2���ڢ��м�ˮ��û���ܣ��رշ�Һ©���������þƾ����ȣ������е��ܿڲ������ȵ����ݣ�ֹͣ���Ⱥ���ĩ���γ�һ��ˮ���ұ���һ��ʱ�䲻�½�����˵�������Ժá���2�֣�
��3��BD��2�֣�
��4��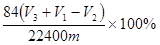 ��2�֣�
��2�֣�
��5����ͬѧ��1�֣���ͬѧ�ķ����У����ɵ�CO2û��ȫ��NaOH��Һ���ա���2�֣�
����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �ⶨʱ��/Сʱ�� | 0 | 1 | 2 | 4 |
| pH | 4.73 | 4.62 | 4.56 | 4.55 |

| 32bc |
| a |
| 32bc |
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡ����ѧ2011��2012ѧ��߶���ѧ����ĩ���Ի�ѧ���� ���ͣ�058
ijѧ��ʵ����ȤС������ͼ1װ������ɡ�NaHCO3��NaCl�������NaHCO3�����IJⶨ����ʵ�飮

(1)�������ӿ�����˳����________(�ýӿ���ĸ��д)��
(2)��װ�����Ӻö�δװҩƷǰ�����������ԵIJ�����________��
(3)��ͬѧ��Ϊ�����������ϴ�������¸Ľ���ʩ������Ϊ���е���________��(�����)
A���ڵ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
B����װ�â���ϡ���ỻ��ϡ���ᣬװ�â���ˮ���ɱ���NaHCO3��Һ
C����װ�â���ˮ���ɱ���Na2CO3��Һ
D���μ�����˹���
(4)��ͬѧ��Ϊ��װ�âڢ�֮��ĵ����ڻ����ˮ��ʹ�ⶨ��������Ӷ�����ͼ2װ�ã��ٶ��ζ�����ʼ����ΪV1 mL�����˶���ΪV2 mL����ע�����ⶨ�ų�������ΪV3 mL(��״����)���������Ʒ����Ϊm g����ԭ�������NaHCO3�����������ı���ʽΪ________��(�ú�V1��V2��V3��m��ʽ�ӱ�ʾ)��

(5)��ͬѧ����ͼ3װ�ã�ͨ���ⶨ�ձ�������������Ϊ�ҡ�����ͬѧ�ķ����У�˭�ķ���������________�������ǣ�________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��12�֣�ijѧ��ʵ����ȤС������ͼ1װ������ɡ�NaHCO3��NaCl�������NaHCO3�����IJⶨ����ʵ�顣

ͼ1
��1���������ӿ�����˳����_________________���ýӿ���ĸ��д����
��2����װ�����Ӻö�δװҩƷǰ�����������ԵIJ�����_________________________��
��3����ͬѧ��Ϊ�����������ϴ�������¸Ľ���ʩ������Ϊ���е���______��������ţ�
A���ڵ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
B����װ�â���ϡ���ỻ��ϡ���ᣬװ�â���ˮ���ɱ���NaHCO3��Һ
C����װ�â���ˮ���ɱ���Na2CO3��Һ
D���μ�����˹���
��4����ͬѧ��Ϊ��װ�âڢ�֮��ĵ����ڻ����ˮ��ʹ�ⶨ��������Ӷ�����ͼ2װ�á��ٶ��ζ�����ʼ����ΪV1mL�����˶���ΪV2mL����ע�����ⶨ�ų�������ΪV3mL����״���£����������Ʒ����Ϊm g����ԭ�������NaHCO3�����������ı���ʽΪ_______��
���ú�V1 ��V2 ��V3 ��m��ʽ�ӱ�ʾ����

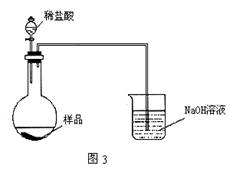
��5����ͬѧ����ͼ3װ�ã�ͨ���ⶨ�ձ�������������Ϊ�ҡ�����ͬѧ�ķ����У�˭�ķ���������__________��������:_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ��ͨ��������ѧ�߶����ϣ����л�ѧ�Ծ��������棩 ���ͣ������
| �ⶨʱ��/Сʱ�� | 1 | 2 | 4 | |
| pH | 4.73 | 4.62 | 4.56 | 4.55 |
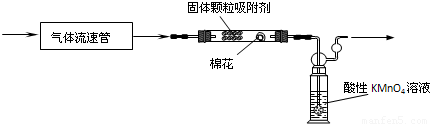
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com