
A”¢B”¢C”¢DĖÄÖÖ·¼Ļć×å»ÆŗĻĪļ¶¼ŹĒijŠ©Ö²Īļ»Ó·¢ÓĶÖŠµÄÖ÷ŅŖ³É·Ö£¬ÓŠµÄŹĒŅ©Īļ£¬ÓŠµÄŹĒĻćĮĻ”£ĖüĆĒµÄ½į¹¹¼ņŹ½ČēĻĀĖłŹ¾£ŗ
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©1 mol BÄÜÓėŗ¬ molBr2µÄäåĖ®·“Ó¦£»CÖŠ¹ŁÄÜĶŵÄĆū³Ę ”£
£Ø2£©ÄÜ·¢ÉśŅų¾µ·“Ó¦µÄÓŠ___________£ØÓĆA”¢B”¢C”¢DĢīæÕ£©”£
£Ø3£©¢ŁDµÄŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåGµÄ½į¹¹¼ņŹ½ČēĻĀ£ŗŠ“³öGÓė×ćĮæNaOHČÜŅŗ¹²ČČ·“Ó¦µÄ»Æѧ·½³Ģ£ŗ ”£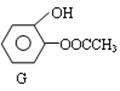
¢ŚDµÄĮķŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåH£¬Ęä±½»·ÉĻÓŠĮ½øöĻąĮŚµÄČ”“ś»ł£¬Ėü¼ČÄÜŹ¹FeCl3ČÜŅŗ±ä×ĻÉ«£¬ÓÖÄÜÓėNaHCO3ČÜŅŗ·“Ó¦·Å³öCO2ĘųĢ壬HµÄ½į¹¹¼ņŹ½ŹĒ ”£
£Ø4£©°“ĻĀĶ¼£¬C¾Ņ»²½·“Ó¦æÉÉś³ÉE£¬EŹĒBµÄĶ¬·ÖŅģ¹¹Ģ壬Ōņ·“Ó¦¢ŁŹōÓŚ_______________ £ØĢī·“Ó¦ĄąŠĶĆū³Ę£©£¬Š“³öFµÄ½į¹¹¼ņŹ½_______________________”£
£Ø1£©4 £Ø2·Ö£© Č©»ł £Ø2·Ö£©
£Ø2£©A”¢C £Ø2·Ö£© £Ø3£©¢Ł 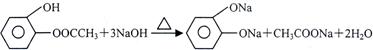 £Ø3·Ö£©
£Ø3·Ö£©
¢Ś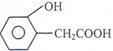 £Ø2·Ö£©
£Ø2·Ö£©
£Ø4£©Ńõ»Æ·“Ó¦ £Ø2·Ö£© 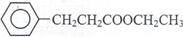 £Ø3·Ö£©
£Ø3·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©øł¾ŻBµÄ½į¹¹æÉÖŖ£¬·ÓōĒ»łµÄĮŚ¶ŌĪ»ÄÜÓėäå·¢ÉśČ”“ś£¬Ģ¼Ģ¼Ė«¼üÓėäå·¢Éś¼Ó³É·“Ó¦£»¹Ź×ī¶ąĻūŗÄ4molBr2,CÖŠµÄ¹ŁÄÜĶÅĪŖČ©»ł”£¢Ę·Ö×ÓÖŠŗ¬ÓŠČ©»łµÄ²ÅÄÜ·¢ÉśŅų¾µ·“Ó¦£¬¹Ź“š°øĪŖA”¢C”£¢Ē¢Ł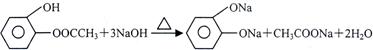 ¢Ś
¢Ś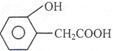 ¢ČC¾Ņ»²½·“Ó¦æÉÉś³ÉE£¬EŹĒBµÄĶ¬·ÖŅģ¹¹Ģ壬Ōņ·“Ó¦¢ŁĪŖŃõ»Æ·“Ó¦£¬EĪŖ£¬·“Ó¦¢ŚĪŖõ„»Æ·“Ó¦£¬FµÄ½į¹¹¼ņŹ½ĪŖC6H5CH2CH2COOCH2CH3.
¢ČC¾Ņ»²½·“Ó¦æÉÉś³ÉE£¬EŹĒBµÄĶ¬·ÖŅģ¹¹Ģ壬Ōņ·“Ó¦¢ŁĪŖŃõ»Æ·“Ó¦£¬EĪŖ£¬·“Ó¦¢ŚĪŖõ„»Æ·“Ó¦£¬FµÄ½į¹¹¼ņŹ½ĪŖC6H5CH2CH2COOCH2CH3.
æ¼µć£ŗæ¼²éÓŠ»śĪļµÄ½į¹¹ÓėŠŌÖŹµČĻą¹ŲÖŖŹ¶”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¾ŪĢ¼Ėįõ„ĪŽÉ«ĶøĆ÷£¬¾ßÓŠÓÅŅģµÄæ¹³å»÷ŠŌ£¬ÄÜÓĆÓŚÖĘŌģÓīŗ½Ō±µÄĆęÕÖ”¢ÖĒÄÜŹÖ»ś»śÉķĶāæĒµČ”£Ė«·Ó»ÆŗĻĪļŹĒŗĻ³É¾ŪĢ¼Ėįõ„µÄµ„ĢåÖ®Ņ»£¬Ä³ÖÖĖ«·Ó»ÆŗĻĪļGµÄŗĻ³ÉĀ·ĻßČēĻĀ£ŗ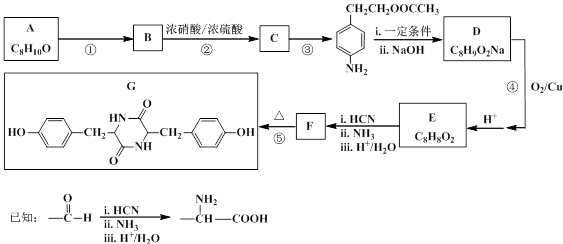
£Ø1£©GÖŠĖłŗ¬µÄ¹ŁÄÜĶųż±½»·Ķā£¬»¹ÓŠ________________”£
£Ø2£©Š“³ö·“Ó¦ĄąŠĶ”£·“Ó¦ ¢Ł ___________£»·“Ó¦ ¢Ū ____________”£
£Ø3£©Š“³ö½į¹¹¼ņŹ½”£A ______________£»F ______________”£
£Ø4£©Š“³ö·“Ó¦ ¢Ü µÄ»Æѧ·½³ĢŹ½_______________________________________”£
£Ø5£©CÓŠ¶ąÖÖĶ¬·ÖŅģ¹¹Ģ壬Š“³öĶ¬Ź±Āś×ćĻĀĮŠĢõ¼žµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½_____________”£
£ØI£©ŹōÓŚ¦Į-°±»łĖį£¬ĒŅ±½»·ÉĻÓŠČżøö»„ĪŖ¼äĪ»µÄČ”“ś»ł
£ØII£©ÓėFeCl3ČÜŅŗ×÷ÓĆĪŽĻŌÉ«ĻÖĻó
£ØIII£©1 moløĆĶ¬·ÖŅģ¹¹ĢåÓė×ćĮæNaOHČÜŅŗ·“Ó¦Ź±£¬×ī¶ąÄÜĻūŗÄ3 mol NaOH
£Ø6£©×ī³£¼ūµÄ¾ŪĢ¼Ėįõ„ŹĒÓĆĖ«·ÓA£Ø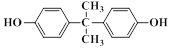 £©Óė¹āĘų£Ø
£©Óė¹āĘų£Ø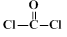 £©¾ŪŗĻµĆµ½£¬ĒėŠ“³öøĆ¾ŪĢ¼Ėįõ„µÄ½į¹¹¼ņŹ½______________________________”£
£©¾ŪŗĻµĆµ½£¬ĒėŠ“³öøĆ¾ŪĢ¼Ėįõ„µÄ½į¹¹¼ņŹ½______________________________”£
ŅŃÖŖ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©Ä³ÓŠ»śĪļAµÄÖŹĮæĪŖ9.2æĖ£¬ĶźČ«Č¼ÉÕŗóÉś³É0.4mol¶žŃõ»ÆĢ¼ŗĶ10.8æĖĖ®£¬ĒŅ“ĖÓŠ»śĪļµÄÕōĘųµÄĻą¶ŌĆܶȏĒĻąĶ¬×“æöĻĀĒāĘųµÄ23±¶£¬Ēó£ŗ
¢Ł“ĖÓŠ»śĪļµÄ·Ö×ÓŹ½ĪŖ
¢ŚŠ“³öøĆÓŠ»śĪļæÉÄܵĽį¹¹¼ņŹ½
£Ø2£©Ä³ĢžBµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ84”£»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁĻĀĮŠĪļÖŹÓėBŅŌČĪŅā±ČĄż»ģŗĻ£¬Čō×ÜĪļÖŹµÄĮæŅ»¶Ø£¬³ä·ÖČ¼ÉÕĻūŗÄŃõĘųµÄĮæ²»ĻąµČµÄŹĒ£ØĢīŠņŗÅ£© ”£
a£®C7H12O2 b£®C6H14 c£®C6H14O d£®C7H14O3
¢ŚČōĮ“ĢžB·Ö×ÓÖŠĖłÓŠµÄĢ¼Ō×Ó¹²Ę½Ćę£¬øĆ·Ö×ÓµÄŅ»ĀČČ”“śĪļÖ»ÓŠŅ»ÖÖ£¬ ŌņBµÄ½į¹¹¼ņŹ½ĪŖ ”£
¢ŪČōĢžB²»ÄÜŹ¹äåĖ®ĶŹÉ«£¬²¢ĒŅĘäŅ»ĀČ“śĪļÖ»ÓŠŅ»ÖÖ£¬ŌņBµÄ½į¹¹¼ņŹ½ĪŖ ”£
£Ø3£©C1£ØŅ»Ģ¼»Æѧ£©ŹĒŅŌŗ¬Ņ»øöĢ¼Ō×ӵĻÆŗĻĪļČē£ŗ¼×Ķé(CH4)”¢ŗĻ³ÉĘų(COŗĶH2)”¢CO2”¢CH3OH”¢HCHOµČĪŖ³õŹ¼·“Ó¦Īļ£¬·“Ó¦ŗĻ³ÉŅ»ĻµĮŠÖŲŅŖµÄ»Æ¹¤ŌĮĻŗĶČ¼ĮĻµÄ»Æѧ”£
¢ŁCOÓėH2°“Ņ»¶Ø±ČĄżæÉŗĻ³ÉŅŅ¶ž“¼£ØC2H6O2£©£¬Ōņn(CO)/n(H2)£½ ”£
¢ŚĘūÓĶĘ½¾ł×é³ÉÓĆCmHn±ķŹ¾£¬ŌņŗĻ³ÉĘūÓĶÓ¦æŲÖĘn(CO)£Æn(H2)£½ ”£(ÓĆm ”¢ n±ķŹ¾)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»ÆŗĻĪļ¢õŹĒ¾ßÓŠæ¹Ńõ»ÆŗĶæ¹Ö×Įö×÷ÓĆ³É·Ö£®»ÆŗĻĪļ¢õŅ²æÉĶعżĶ¼ĖłŹ¾·½·ØŗĻ³É£ŗ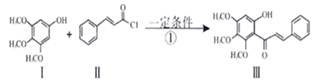
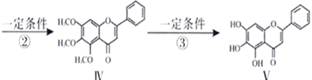
ŅŃÖŖ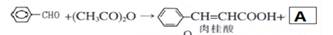 ”¢
Ӣ
£Ø1£©·“Ó¦¢ŁµÄĄąŠĶ
£Ø2£©»ÆŗĻĪļ¦©µÄ·Ö×ÓŹ½
£Ø3£©¢ŁAµÄ½į¹¹¼ņŹ½ ”£
¢Ś1molV×ī¶ąĻūŗÄNaOH ”£1molČā¹šĖį×ī¶ąĻūŗÄH2 mol£Ø1·Ö£©
£Ø4£©»ÆŗĻĪļIŗĶ¢ņ·“Ó¦»¹æÉŅŌµĆµ½Ņ»ÖÖõ„£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø5£©¹ŲÓŚ¢õµÄĖµ·ØÕżČ·µÄŹĒ( )
| A£®·Ö×ÓÖŠÓŠČżøö±½»· |
| B£®ÄÜ·¢ÉśĖ®½ā·“Ó¦ |
| C£®Ź¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ« |
| D£®ÓėFeCl3·¢ÉśĻŌÉ«·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖĢžB·Ö×ÓÄŚC”¢HŌ×ÓøöŹż±ČĪŖ1©U2£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖ28£¬ŗĖ“Ź²ÕńĒāĘ×ĻŌŹ¾·Ö×ÓÖŠÖ»ÓŠŅ»ÖÖ»Æѧ»·¾³µÄĒāŌ×Ó£¬ĒŅÓŠČēĻĀµÄ×Ŗ»Æ¹ŲĻµ£ŗ
£Ø1£©BµÄ½į¹¹¼ņŹ½ŹĒ
£Ø2£©·“Ó¦¢ŁŹĒDÓėHCl°“ĪļÖŹµÄĮæÖ®±Č1©U1µÄ»ÆŗĻ·“Ó¦£¬ŌņDµÄ·Ö×ÓŹ½ŹĒ
·“Ó¦¢ŚæɱķŹ¾ĪŖ£ŗG + NH3 ”ś F + HCl (Ī“ÅäĘ½)£¬øĆ·“Ó¦ÅäĘ½ŗóµÄ»Æѧ·½³ĢŹ½ŹĒ£ØÓŠ»ś»ÆŗĻĪļ¾łÓĆ½į¹¹¼ņŹ½±ķŹ¾£©£ŗ
»ÆŗĻĪļE£ØHOCH2CH2Cl£©ŗĶ F [ HN(CH2CH3)2 ]ŹĒŅ©Ę·ĘÕĀ³æØŅņŗĻ³ÉµÄÖŲŅŖÖŠ¼äĢ壬ĘÕĀ³æØŅņµÄŗĻ³ÉĀ·ĻßČēĻĀ£ŗ£ØŅŃÖŖ£ŗ £©
£©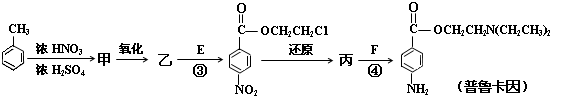
£Ø3£©¼×µÄ½į¹¹¼ņŹ½ŹĒ £Ø1·Ö£©”£Óɼױ½Éś³É¼×µÄ·“Ó¦ĄąŠĶŹĒ £Ø1·Ö£©
£Ø4£©ŅŅÖŠÓŠĮ½ÖÖŗ¬Ńõ¹ŁÄÜĶÅ£¬·“Ó¦¢ŪµÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ
£Ø5£©ĘÕĀ³æØŅņÓŠĮ½ÖÖĖ®½ā²śĪļ¶”ŗĶĪģ
¢ŁĪģÓė¼×»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬ĪģµÄ½į¹¹¼ņŹ½ŹĒ £Ø1·Ö£©
¢ŚĪģ¾¾ŪŗĻ·“Ó¦ÖĘ³ÉµÄøß·Ö×ÓĻĖĪ¬¹ć·ŗÓĆÓŚĶØѶ”¢Óīŗ½µČĮģÓņ£®øĆ¾ŪŗĻ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ
¢ŪDµÄŗģĶā¹āĘ×±ķĆ÷·Ö×ÓÄŚ³żC”ŖH¼ü”¢C”ŖC¼üĶā»¹ŗ¬ÓŠĮ½øöC”ŖOµ„¼ü£®ŌņDÓėFŌŚŅ»¶ØĢõ¼žĻĀ·“Ӧɜ³É¶”µÄ»Æѧ·½³ĢŹ½ŹĒ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖÓŠ»śĪļAÓÉC”¢H”¢OČżÖÖŌŖĖŲ×é³É£¬Č”1 mol ³ä·ÖČ¼ÉÕÉś³É4 mol CO2ŗĶ54 g H2O£¬A µÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ86”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AĪļÖŹµÄ·Ö×ÓŹ½ŹĒ ”£
£Ø2£©AĪļÖŹ·ūŗĻĻĀĮŠĢõ¼ž£ŗ
¢Ł ÄÜÓėBr2µÄCCl4ČÜŅŗ·¢Éś»Æѧ·“Ó¦Ź¹ĘäĶŹÉ«£»¢Ś ÄÜÓėNaHCO3ČÜŅŗ·“Ó¦·Å³öCO2ĘųĢ壻
·ūŗĻÉĻŹöŅŖĒóµÄAÓŠ¶ąÖÖ£¬Ęä½į¹¹¼ņŹ½·Ö±šŹĒ ”££Ø²»ŅŖĒóĖ³·“Ņģ¹¹ÓėĮ¢ĢåŅģ¹¹£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖ£ŗ

ĪŖŗĻ³ÉijÖÖŅŗ¾§²ÄĮĻµÄÖŠ¼äĢåM£¬ÓŠČĖĢį³öČēÉĻ²»Ķ¬µÄŗĻ³ÉĶ¾¾¶
£Ø1£©³£ĪĀĻĀ£¬ĻĀĮŠĪļÖŹÄÜÓėA·¢Éś·“Ó¦µÄÓŠ £ØĢīŠņŗÅ£©
a.±½ b.Br2/CCl4 c.ŅŅĖįŅŅõ„ d.KMnO4/H+ČÜŅŗ
£Ø2£©MÖŠ¹ŁÄÜĶŵÄĆū³ĘŹĒ £¬ÓÉC”śB·“Ó¦ĄąŠĶĪŖ ”£
£Ø3£©ÓÉA“߻ƼÓĒāÉś³ÉMµÄ¹ż³ĢÖŠ£¬æÉÄÜÓŠÖŠ¼äÉś³ÉĪļŗĶ £ØŠ“½į¹¹¼ņŹ½£© ”£
£Ø4£©¼ģŃéBÖŠŹĒ·ńŗ¬ÓŠCæÉŃ”ÓƵďŌ¼ĮŹĒ £ØČĪŠ“Ņ»ÖÖĆū³Ę£©”£
£Ø5£©ĪļÖŹBŅ²æÉÓÉC10H13ClÓėNaOHĖ®ČÜŅŗ¹²ČČÉś³É£¬Š“³öÓÉC10H13ClŗĶNaOHĖ®ČÜŅŗ¹²ČČ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø6£©CµÄŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåE¾ßÓŠČēĻĀĢŲµć£ŗ
a.·Ö×ÓÖŠŗ¬”ŖOCH2CH3 b.±½»·ÉĻÖ»ÓŠĮ½ÖÖ»Æѧ»·¾³²»Ķ¬µÄĒāŌ×Ó
Š“³öEŌŚŅ»¶ØĢõ¼žĻĀ·¢Éś¼Ó¾Ū·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ij·¼ĻćĢžŃÜÉśĪļµÄ·Ö×ÓŹ½ĪŖC7H8O£¬øł¾ŻĻĀĮŠŹµŃéĻÖĻó£¬Č·¶Ø½į¹¹¼ņŹ½”£
£Ø1£©ČōøĆÓŠ»śĪļÓöFeCl3ČÜŅŗĻŌÉ«£¬ŌņĘäæÉÄܵĽį¹¹¼ņŹ½ĪŖ£ŗ
£¬ £¬ ”£
ÉĻŹöµÄČżÖÖĪļÖŹÖŠ£¬±½»·ÉĻµÄŅ»ĀČ“śĪļÖÖĄą×īÉŁµÄŅ»ĀČ“śĢžÓŠ ÖÖ£¬×ī¶ąµÄŅ»ĀČ“śĢžÓŠ ÖÖ”£
£Ø2£©ČōøĆÓŠ»śĪļ²»ÄÜÓė½šŹōÄĘ·“Ó¦²śÉśĒāĘų£¬Ōņ½į¹¹ÖŠ»įÓŠ £ØĢī¹ŁÄÜĶÅ£©³öĻÖ”£
£Ø3£©ČōøĆÓŠ»śĪļÓöFeCl3ČÜŅŗ²»ĻŌÉ«£¬µ«Óė½šŹōÄĘ·“Ó¦·Å³öH2£¬ŌņĘä·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©ÄÜÓĆĄ“¼ų±šµ°°×ÖŹ”¢µķ·ŪŗĶ·ŹŌķĖ®µÄŅ»ÖÖĪļÖŹŹĒ ”£
£Ø2£©ÖĘŌģĘÕĶز£Į§µÄÖ÷ŅŖŌĮĻŹĒ£ŗ“æ¼ī”¢ŹÆ»ŅŹÆŗĶŹÆÓ¢£¬ŌŚ²£Į§Ņ¤ÖŠĒæČČŹ±µÄÖ÷ŅŖ·“Ó¦ŹĒ £¬ ”£
£Ø3£©ĖÜĮĻ”¢Ļš½ŗ”¢¹āµ¼ĻĖĪ¬ŹĒĻÖ“śÉś»īÖŠ³£ÓĆµÄ²ÄĮĻ”£ÉĻŹöČżÖÖ²ÄĮĻÖŠŹōÓŚÓŠ»śøß·Ö×Ó²ÄĮĻµÄŹĒ ”£
¹āµ¼ĻĖĪ¬µÄ»Æѧ³É·ÖŹĒ£ØĢī»ÆѧŹ½£© ”£
¢Č°¢Ė¾Ę„ĮÖÓÖ³ĘŅŅõ£Ė®ŃīĖį£¬ŹĒŅ»ÖÖ½āČČ”¢ÕņĶ“”¢ĻūŃ×Ņ©Īļ”£°¢Ė¾Ę„ĮÖ·Ö×ÓÖŠŗ¬4.5%Ēā”¢35.5%Ńõ”¢60%Ģ¼£¬ĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ180”£
°¢Ė¾Ę„ĮÖ·Ö×ÓŹ½CxHyOzÖŠ£¬x”Ćy”Ćz= ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com