
 mL��
mL�� ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
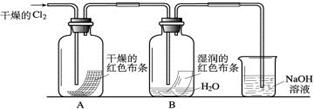
| A������KOH����ʱ��ֱ����ֽ������ |
| B��KOH�����ܽ��δ��ȫ��ȴ��ת��������ƿ�У� |
| C������ʱ����������ƿ���ߣ� |
| D��������Һǰ������ƿδ��ȫ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
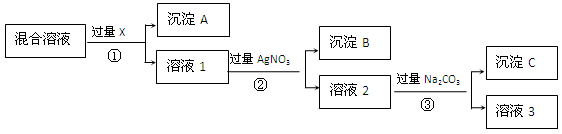
 ���У�Ȼ����ע��10 mL CCl4���Ǻò�������������������̨�ϣ��ȷֲ��ȡ�ϲ�Һ
���У�Ȼ����ע��10 mL CCl4���Ǻò�������������������̨�ϣ��ȷֲ��ȡ�ϲ�Һ ���²�Һ���ʻ���ɫ����________(��ϲ�Һ�����²�Һ��)����ʹ��ɫ������ɫ����________(��ϲ�Һ�����²�Һ��)��
���²�Һ���ʻ���ɫ����________(��ϲ�Һ�����²�Һ��)����ʹ��ɫ������ɫ����________(��ϲ�Һ�����²�Һ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ���¿̶ȡ�
���¿̶ȡ� Щʵ��
Щʵ�� ��������� ��
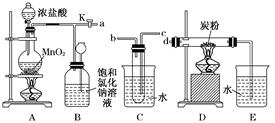
��������� ��| A��ת�ƴ���Һ������ƿʱ��δϴ���ձ� |
| B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ���� |
| C���ζ�ʱ����Ӧ����ҡ��̫���ң�����������Һ���� |
| D���ζ����յ�ʱ���ζ��ܼ�������Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
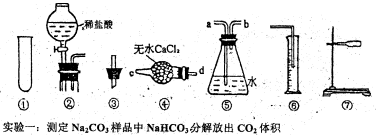
 12�֣���̼���ƣ�Na2CO4����ϴ�ӡ�ӡȾ����ֽ��ҽҩ�����������д���Ӧ�á�
12�֣���̼���ƣ�Na2CO4����ϴ�ӡ�ӡȾ����ֽ��ҽҩ�����������д���Ӧ�á� 2H2O2 ��2H2O+ O2��.Ϊ�ⶨ�ѱ��ʵĹ�̼����(��̼����)�Ĵ��ȣ������ͼ��ʾ��ʵ�飺QΪ���������õĵ��Ե������뷴Ӧ��������ﷴӦ������ȡһ��������Ʒ�������������̷������У���ͼ��װ��ʵ��װ�ã���Һ©���Ļ�������ϡH2SO4���������С�
2H2O2 ��2H2O+ O2��.Ϊ�ⶨ�ѱ��ʵĹ�̼����(��̼����)�Ĵ��ȣ������ͼ��ʾ��ʵ�飺QΪ���������õĵ��Ե������뷴Ӧ��������ﷴӦ������ȡһ��������Ʒ�������������̷������У���ͼ��װ��ʵ��װ�ã���Һ©���Ļ�������ϡH2SO4���������С�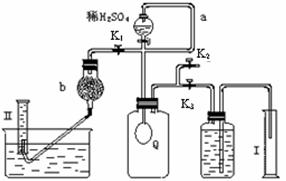
 ____________
____________ _____��
_____��| A������������Q�͵������У�δȫ��������Ͳ�� |
B����Ͳ�����ʱ ����ͲҺ�����ˮ��Һ�� ����ͲҺ�����ˮ��Һ�� |
| C���Ҳ���Ͳ��ʹ�Һ����ƿ���ӵ����ڵ�Һ��û�м������x |
| D�����������ֵx��yû�п۳��μӵ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������ʳ�üӵ���(��KIO3)�к��е� |
| B���õ�Ƽ����������Ƿ��в������� |
| C���ᴿ�����ʿ����ڵ�������Һ�мӱ���CuSO4��Һ��������������Ȼ��ѳ�����������ˮ�� |
| D��������Һ�м����������ˮ�⣬��ȴ����������Һ��������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com