
��ͼ��ʾ������ƿ�зֱ�װ�뺬��̪��0.01 mol·L��1 CH3COONa��Һ�����ֱ������ʢ��ˮ���ձ��У�Ȼ�����ձ����м�����ʯ�ң����ձ����м���NH4NO3���壬�ձ����в����κ����ʡ�
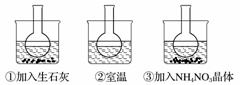
(1)����̪��0.01 mol·L��1 CH3COONa��Һ��dz��ɫ��ԭ��Ϊ__________________________
(�����ӷ���ʽ�ͱ�Ҫ���ֽ���)��
(2)ʵ������з�����ƿ������Һ��ɫ�����ƿ������Һ��ɫ��dz��������������ȷ����________(����ĸ)��
A��ˮ�ⷴӦΪ���ȷ�Ӧ
B��ˮ�ⷴӦΪ���ȷ�Ӧ
C��NH4NO3����ˮʱ�ų�����
D��NH4NO3����ˮʱ��������
(3)��0.01 mol·L��1 CH3COONa��Һ�зֱ����NaOH���塢Na2CO3���塢FeSO4���壬ʹCH3COO��ˮ��ƽ���ƶ��ķ���ֱ�Ϊ____________��____________��____________��(������ҡ����ƶ���)
�𰸡�(1)CH3COO����H2O??CH3COOH��OH����ʹ��Һ�Լ��ԡ�(2)BD��(3)������
������(1)CH3COONa��CH3COO��ˮ��ʹ��Һ�Լ��ԣ���̪��Һ�����Ժ�ɫ��
(2)��ʯ����ˮ���ҷ�Ӧ�ų������ȣ�������ƿ������Һ��ɫ����ж�ˮ��ƽ�������ƶ���˵��ˮ�ⷴӦ�����ȷ�Ӧ��ͬʱ��ƿ������Һ��ɫ��dz����NH4NO3����ˮʱ����������
(3)������CH3COO����ˮ�⣻CO ˮ���Լ��ԣ���CH3COO����ˮ������ƣ�Fe2��ˮ�������ԣ���CH3COO����ˮ����ٽ���
ˮ���Լ��ԣ���CH3COO����ˮ������ƣ�Fe2��ˮ�������ԣ���CH3COO����ˮ����ٽ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̬�����Ի�̬ԭ�ӵ�ԭ�Ӻ�������Ų��������±仯����������������(����)
A.1s22s22p63s23p2��1s22s22p63s23p1
B.1s22s22p63s23p3��1s22s22p63s23p2
C.1s22s22p63s23p4��1s22s22p63s23p3
D.1s22s22p63s23p63d104s24p2��1s22s22p63s23p63d104s24p1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����1��20��Ԫ��A��B��C��D����Ӧ�����ʵ����ʻ����ṹ���±���
| Ԫ�� | �������ʻ����ṹ |
| A | M������2�ԳɶԵ��� |
| B | B��������D�����Ӿ�����ͬ���Ӳ�ṹ���ҿ��������γɸ���� |
| C | �����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
| D | Ԫ����������ǣ�7�� |
(1)Ԫ��A��ԭ������㹲��________�ֲ�ͬ�˶�״̬�ĵ��ӣ���________��������ͬ�ĵ��ӡ�B��������D�����������γɵĸ�����Ļ�ѧʽ��________��
(2)Ԫ��C����Ԫ���γɴ�һ����λ����ɵ����ӣ�д�������ĵ���ʽ________________________________________________________________________(��Ԫ�ط��ű�ʾ)��
(3)Ԫ��A��Ԫ��D��ȣ��ǽ����Խ�ǿ����________(��Ԫ�ط��ű�ʾ)�����б�������֤����һ��ʵ����________��
A��������A�ĵ��ʺ�D�ĵ���״̬��ͬ
B��A���⻯���D���⻯���ȶ�
C��һ��������D�ܴ�A���⻯��ˮ��Һ���û���A����
D��HD�����Ա�HA����ǿ
(4)C���⻯���̬ʱ����________���壬���⻯����A������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽ��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͬ�¶ȡ���ͬŨ���µİ�����Һ����pH��С�����˳����ͼ��ʾ��ͼ�Т٢ڢۢܢݴ��������ʿ��ֱܷ�Ϊ(����)
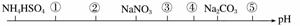
A��NH4Cl��(NH4)2SO4��CH3COONa��NaHCO3��NaOH
B��(NH4)2SO4��NH4Cl��CH3COONa��NaHCO3��NaOH
C��(NH4)2SO4��NH4Cl��NaOH��CH3COONa NaHCO3
D��CH3COOH��NH4Cl��(NH4)2SO4��NaHCO3��NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)̼�����Һ���ɵõ��Ĺ���������___________________________________________��
ԭ����________________________________________________________________________��
(2)KAl(SO4)2��Һ���ɵõ��Ĺ���������__________��ԭ����_______________��
(3)FeCl2��Һ�������յõ��Ĺ���������__________��ԭ����______________________��
(4)̼��������Һ�������յõ��Ĺ���������________��ԭ����_________________________
________________________________________________________________________��
(5)����������Һ�������յõ��Ĺ���������________��ԭ����__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
25 ��ʱ��H2SO3??HSO ��H���ĵ��볣��Ka��1��10��2mol·L��1������¶���NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kh��______mol·L��1������NaHSO3��Һ�м���������I2������Һ��
��H���ĵ��볣��Ka��1��10��2mol·L��1������¶���NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kh��______mol·L��1������NaHSO3��Һ�м���������I2������Һ��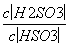 ��________(���������С�����䡱)��
��________(���������С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д�����е������ʵĵ��뷽��ʽ
(1)H2SO4_______________________________________________________________________��
(2)H2CO3_______________________________________________________________________��
(3)Ca(OH)2____________________________________________________________________��
(4)Fe(OH)3_____________________________________________________________________��
(5)NH3·H2O____________________________________________________________________��
(6)NaCl________________________________________________________________________��
(7)BaSO4______________________________________________________________________��
(8)NaHSO4_____________________________________________________________________��
(9)NaHCO3_____________________________________________________________________��
(10)NaHSO4(����)______________________________________________________________��
(11)Al2O3(����)_______________________________________________________________��
(12)CH3COOH_________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ǻ��ֱ�����л������������ɵĻ������У����ڴ������________�����ڷ������________(����ĸ)��
A��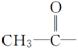 B��C6H5—
B��C6H5—
C��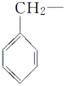 D��
D��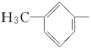
E��HO—CH2—CH2—
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com