
��Ҫ��д��298K��101 kPaʱ���з�Ӧ���Ȼ�ѧ����ʽ��
��1��3mol NO2(g)��1 mol H2O(1)��Ӧ����HNO3(aq)��NO(g)������138kJ��____________��
��2��1 mol HgO(s)�ֽ�ΪҺ̬��������������90.7kJ��___________________��
��3������ͼ��һ����������ȫȼ������CO2��1 mol H2O(l)�����е������仯ͼ�����ڸ�ͼ�е����������롰����������
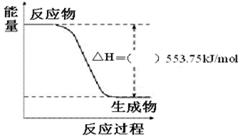
��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��_______________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �� | |||||||||||||||||
| �� | �� | �� | �� | �� | |||||||||||||
| �� | �� | ||||||||||||||||
| �� | �� |

| M | ||
4
|
| M | ||
4
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣���Ҫ��д��298K��101 kPaʱ���з�Ӧ���Ȼ�ѧ����ʽ��
��1��3mol NO2(g)��1 mol H2O(1)��Ӧ����HNO3(aq)��NO(g)������138kJ��____________��
��2��1 mol HgO(s)�ֽ�ΪҺ̬��������������90.7kJ��___________________��
��3������ͼ��һ����������ȫȼ������CO2��1 mol H2O(l)�����е������仯ͼ�����ڸ�ͼ�е����������롰����������
��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�Ƹ���ѧ�߶���ѧ�����п������⻯ѧ ���ͣ������
��8�֣���Ҫ��д��298K��101 kPaʱ���з�Ӧ���Ȼ�ѧ����ʽ��
��1��3mol NO2(g)��1 mol H2O(1)��Ӧ����HNO3(aq)��NO(g)������138kJ��____________��
��2��1 mol HgO(s)�ֽ�ΪҺ̬��������������90.7kJ��___________________��
��3������ͼ��һ����������ȫȼ������CO2��1 mol H2O(l)�����е������仯ͼ�����ڸ�ͼ�е����������롰����������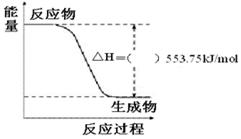
��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�߶���ѧ�����п������⻯ѧ ���ͣ������
��8�֣���Ҫ��д��298K��101 kPaʱ���з�Ӧ���Ȼ�ѧ����ʽ��
��1��3mol NO2(g)��1 mol H2O(1)��Ӧ����HNO3(aq)��NO(g)������138kJ��____________��
��2��1 mol HgO(s)�ֽ�ΪҺ̬��������������90.7kJ��___________________��
��3������ͼ��һ����������ȫȼ������CO2��1 mol H2O(l)�����е������仯ͼ�����ڸ�ͼ�е����������롰����������

��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��_______________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com