
��ҵ�ϳ�����������ʢװ��Ũ���ᣮΪ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС�����������̽�����
(1)����ȥ�����������������(̼�ظ�)������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ����________��
(2)����ȡ����6.0 g����15.0 mlŨ�����У����ȣ����Ӧ��õ���ҺX���ռ�������Y��
�ټ�ͬѧ��ΪX�г�Fe3+����ܺ���Fe2+��д������Fe2+���п��ܵ����ӷ�Ӧ����ʽ��________��
��Ҫȷ��������Fe2+��Ӧѡ��________(ѡ�����)��
a��KSCN��Һ����ˮ
b�����ۺ�KSCN��Һ
c��Ũ��ˮ
d������KMnO4��Һ
����ͬѧȡ336 ml(��״��)����Yͨ��������ˮ�У�������Ӧ�Ļ�ѧ����ʽΪ��________��
Ȼ���������BaCl2��Һ�����ʵ�������ø������2.33 g�����ڴ���֪����Y��SO2���������Ϊ________��
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣���ҵ�ϳ�����������ʢװ��Ũ�ᡣΪ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС�����������̽�����
��1������ȥ�������������������̼�ظ֣�������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ���� ��
��2������ȡ����6.0g����15.0mLŨ�����У����ȣ����Ӧ��õ���ҺX���ռ�������Y��
��ͬѧ��ΪX�г�Fe3+����ܺ���Fe2+����Ҫȷ�����д���Fe2+����Ҫ���е�ʵ�����������ͽ����� ��
��ͬѧ��Ϊ����Y�г�����SO2��H2�⣬�����ܺ���CO2���塣Ϊ�����������̽��ʵ��װ�á�ͼ�мг�����ʡ�ԣ�M��ʢ�г���ʯ��ˮ��
����Ϊ����Y�л�����CO2�������� ���û�ѧ����ʽ������
��װ��A���Լ��������� ��
��Ϊȷ��CO2�Ĵ��ڣ�����װ��������M�� ��ѡ����ţ���
a��A֮ǰ b��A-B�� c��B-C�� d��C-D��
����˵������Y�к���H2��ʵ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ����11���¿������ۺ����⣨��ѧ���֣� ���ͣ�ʵ����
��16�֣���ҵ�ϳ�����������ʢװ��Ũ�ᡣΪ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС�����������̽�����
��1������ȥ�������������������̼�ظ֣�������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ���� ��
��2������ȡ����6.0g����15.0mLŨ�����У����ȣ����Ӧ��õ���ҺX���ռ�������Y��
��ͬѧ��ΪX�г�Fe3+����ܺ���Fe2+����Ҫȷ�����д���Fe2+����Ҫ���е�ʵ�����������ͽ����� ��
��ͬѧ��Ϊ����Y�г�����SO2��H2�⣬�����ܺ���CO2���塣Ϊ�����������̽��ʵ��װ�á�ͼ�мг�����ʡ�ԣ�M��ʢ�г���ʯ��ˮ��
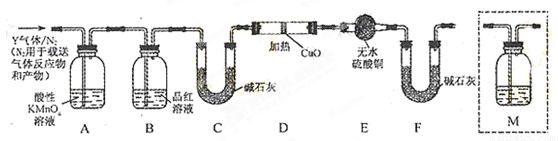
����Ϊ����Y�л�����CO2�������� ���û�ѧ����ʽ������
��װ��A���Լ��������� ��
��Ϊȷ��CO2�Ĵ��ڣ�����װ��������M�� ��ѡ����ţ���
a��A֮ǰ b��A-B�� c��B-C�� d��C-D��
����˵������Y�к���H2��ʵ�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com