|
|
��֪��CH3��CH��CH2��HBr��CH3��CHBr��CH3
1molij��A���ȼ�պ���Եõ�8molCO2��4molH2O������A�ڲ�ͬ�������ܷ�����ͼ��ʾ��һϵ�б仯��
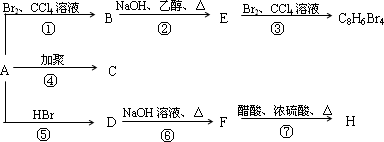
| (1) |
|
A�Ļ�ѧʽ��________��A�Ľṹ��ʽ��________
|
|
(2) |
|
������Ӧ�У�����________��Ӧ������________��Ӧ��(�Ӧ���)
|
|
(3) |
|
д��C��D��E��H���ʵĽṹ��ʽ��
C________��D________��E________��H________��
|
|
(4) |
|
д��D��F��Ӧ�Ļ�ѧ����ʽ________
|
|
|
�𰸣�
������
(1) |
C8H8��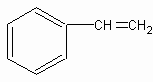 (ÿ��1��) (ÿ��1��)
|
(2) |
�ӳɣ�����(��ȡ��)��(ÿ��1��)
|
(3) |
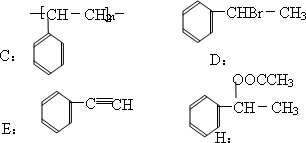
|
(4) |
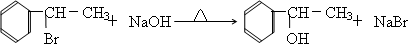
|
��ϰ��ϵ�д�
���ϰ��
��Ŀ�����л�ѧ
��Դ����һ��ѧ����3�¡��л�������Ļ����Ӧ�á�3.1.2��ʯ�����ơ���ϩ(�ս̰����2) �ս̰�
���ͣ�013
|
��֪�л���CH3��CH��CH��C��C��CH��CH2
������˵����ȷ����
|
| [����] |
A�� |
����ԭ�ӿ�����ͬһƽ����
|
B�� |
����̼ԭ�ӿ�����ͬһƽ����
|
C�� |
�����14��ԭ����ͬһƽ����
|
D�� |
����̼ԭ��һ����ͬһƽ����
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ���麣�кͷ���ѧ2007������¿���ѧ�Ծ�
���ͣ�022
|
|
��֪��CH3��CH��CH2��HBr��CH3��CHBr��CH3
1molij��A���ȼ�պ���Եõ�8molCO2��4molH2O������A�ڲ�ͬ�������ܷ�����ͼ��ʾ��һϵ�б仯��
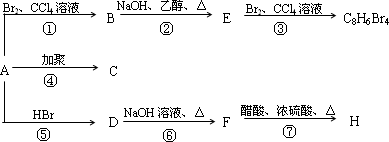
| (1) |
|
A�Ļ�ѧʽ��________��A�Ľṹ��ʽ��________
|
|
(2) |
|
������Ӧ�У�����________��Ӧ������________��Ӧ��(�Ӧ����)
|
|
(3) |
|
д��C��D��E��H���ʵĽṹ��ʽ��
C________��D________��
E________��H________��
|
|
(4) |
|
д��D��F��Ӧ�Ļ�ѧ����ʽ________
|
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ��2006��2007����ƽ����ѧ��������(��)����ѧ�Ծ�
���ͣ�022
|
|
��֪��CH3��CH��CH2 ��HBr��CH3��CHBr��CH3
1molij��A���ȼ�պ���Եõ�8 mol CO2��4 mol H2O������A �ڲ�ͬ����
���ܷ�����ͼ��ʾ��һϵ�б仯��
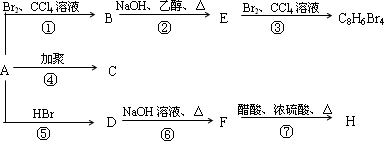
| (1) |
|
������Ӧ�У�����________��Ӧ������________��Ӧ��(�Ӧ���)
|
|
(2) |
|
д��C��D��E��H���ʵĽṹ��ʽ��
C________��D________��
E________��H________��
|
|
(3) |
|
д��D �� F��Ӧ�Ļ�ѧ����ʽ________��
|
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ
��Դ���ͷ���ѧ2007������¿���ѧ�Ծ�
���ͣ�022
|
|
��֪��CH3��CH��CH2��HBr��CH3��CHBr��CH3
1molij��A���ȼ�պ���Եõ�8molCO2��4molH2O������A�ڲ�ͬ�������ܷ�����ͼ��ʾ��һϵ�б仯��

| (1) |
|
A�Ļ�ѧʽ��________A�Ľṹ��ʽ��________
|
|
(2) |
|
������Ӧ�У�����________��Ӧ������________��Ӧ��(�Ӧ����)
|
|
(3) |
|
д��C��D��E��H���ʵĽṹ��ʽ��
C________��D________��
E________��H________��
|
|
(4) |
|
д��D��F��Ӧ�Ļ�ѧ����ʽ________
|
|
|
�鿴�𰸺ͽ���>>

 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�