
ĒėĶź³ÉĻĀĮŠĪŹĢā£ŗ
(1)Ļą¶Ō·Ö×ÓÖŹĮæĪŖM2µÄõ„µÄ·Ö×ÓŹ½ĪŖ______________________________”£
(2)±„ŗĶŅ»ŌŖōČĖįµÄ·Ö×ÓŹ½·Ö±šĪŖ______________________£¬______________________”£
(3)²Ī¼Ó·“Ó¦µÄ“¼µÄ½į¹¹¼ņŹ½ĪŖ_______________”¢_______________”¢_______________”¢_______________(²»±ŲĢīĀś)”£
(4)ÓėĻą¶Ō·Ö×ÓÖŹĮæĪŖM1µÄõ„»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬ÄÜ·¢ÉśŅų¾µ·“Ó¦£¬ÄÜÓė½šŹōÄĘ·“Ó¦²śÉśĒāĘųµÄÓŠ»śĪļµÄĶ¬·ÖŅģ¹¹ĢåÓŠ_______________ÖÖ”£
(1)C5H10O2
(2)C2H4O
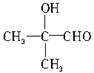
(3)CH3CH2CH2OHӢ![]() ӢCH3CH2OH
ӢCH3CH2OH
(4)5
½āĪö£ŗøł¾Ż±„ŗĶõ„µÄĶØŹ½CnH2nO2£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖM2µÄõ„ÖŠµÄŃõµÄÖŹĮæ·ÖŹżĪŖ31.4%£¬ŌņÓŠ£ŗ
![]() ”Į100%=31.4% n=5,¼“øĆĪļÖŹµÄ·Ö×ÓŹ½ĪŖC5H10O2£¬ÓÉÓŚøĆĪļÖŹÖ»ÓŠČżÖÖõ„µÄĶ¬·ÖŅģ¹¹Ģ壬M1”¢M2”¢M3ŅĄ“ĪŌö“ó14£¬¹ŹÖ»ÄÜŹĒCH3CH2COOC2H5”¢CH3COOCH2CH2CH3”¢CH3COOCH(CH3)2£¬¶ŌÓ¦µÄōČĖį·Ö±šŹĒCH3CH2COOHŗĶCH3COOH£¬¶ŌÓ¦µÄ“¼·Ö±šŹĒC2H5OH”¢CH3CH2CH2OH”¢
”Į100%=31.4% n=5,¼“øĆĪļÖŹµÄ·Ö×ÓŹ½ĪŖC5H10O2£¬ÓÉÓŚøĆĪļÖŹÖ»ÓŠČżÖÖõ„µÄĶ¬·ÖŅģ¹¹Ģ壬M1”¢M2”¢M3ŅĄ“ĪŌö“ó14£¬¹ŹÖ»ÄÜŹĒCH3CH2COOC2H5”¢CH3COOCH2CH2CH3”¢CH3COOCH(CH3)2£¬¶ŌÓ¦µÄōČĖį·Ö±šŹĒCH3CH2COOHŗĶCH3COOH£¬¶ŌÓ¦µÄ“¼·Ö±šŹĒC2H5OH”¢CH3CH2CH2OH”¢![]() ”£Ļą¶Ō·Ö×ÓÖŹĮæĪŖM1µÄĪļÖŹµÄ·Ö×ÓŹ½ĪŖC4H8O2£¬·ūŗĻĢāøųĢõ¼žµÄøĆÓŠ»śĪļµÄĶ¬·ÖŅģ¹¹ĢåÓŠ
”£Ļą¶Ō·Ö×ÓÖŹĮæĪŖM1µÄĪļÖŹµÄ·Ö×ÓŹ½ĪŖC4H8O2£¬·ūŗĻĢāøųĢõ¼žµÄøĆÓŠ»śĪļµÄĶ¬·ÖŅģ¹¹ĢåÓŠ
![]() Ӣ
Ӣ![]() Ӣ
Ӣ![]() Ӣ
Ӣ![]() Ӣ
”¢ ¹²5ÖÖ”£
¹²5ÖÖ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø1£©Ļą¶Ō·Ö×ÓÖŹĮæĪŖM2µÄõ„µÄ·Ö×ÓŹ½ĪŖ____________”£
£Ø2£©±„ŗĶŅ»ŌŖōČĖįµÄ»ÆѧŹ½·Ö±šĪŖ____________”¢____________________________________”£
£Ø3£©²Ī¼Ó·“Ó¦µÄ“¼½į¹¹¼ņŹ½ĪŖ____________”¢____________”¢____________”¢____________£Ø²»±ŲĢīĀś£©”£
£Ø4£©ÓėĻą¶Ō·Ö×ÓÖŹĮæĪŖM1µÄõ„»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬ÄÜ·¢ÉśŅų¾µ·“Ó¦”¢ÄÜÓė½šŹōÄĘ·“Ó¦²śÉśĒāĘųµÄÓŠ»śĪļµÄĶ¬·ÖŅģ¹¹ĢåÓŠ____________ÖÖ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠ±„ŗĶŅ»ŌŖ“¼µÄ»ģŗĻĪļŗĶ±„ŗĶŅ»ŌŖōČĖįµÄ»ģŗĻĪļŌŚŅ»¶ØĢõ¼žĻĀ·“Ӧɜ³É¶ąÖÖõ„µÄ»ģŗĻĪļ£¬õ„µÄĻą¶Ō·Ö×ÓÖŹĮæ·Ö±šĪŖM1”¢M2”¢M3£¬²¢ŅĄ“ĪŌö¼Ó14”£¾·ÖĪö£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖM2µÄõ„£¬ĘäÖŠŃõŌŖĖŲĖłÕ¼µÄÖŹĮæ·ÖŹżĪŖ31.4%£¬²¢ÓŠČżÖÖõ„µÄĶ¬·ÖŅģ¹¹Ģ唣
ĒėĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©Ļą¶Ō·Ö×ÓÖŹĮæĪŖM2µÄõ„µÄ·Ö×ÓŹ½ĪŖ______________________________”£
£Ø2£©±„ŗĶŅ»ŌŖōČĖįµÄ·Ö×ÓŹ½·Ö±šĪŖ______________________£¬______________________”£
£Ø3£©²Ī¼Ó·“Ó¦µÄ“¼µÄ½į¹¹¼ņŹ½ĪŖ_______________”¢_______________”¢_______________”¢_______________£Ø²»±ŲĢīĀś£©”£
£Ø4£©ÓėĻą¶Ō·Ö×ÓÖŹĮæĪŖM1µÄõ„»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬ÄÜ·¢ÉśŅų¾µ·“Ó¦£¬ÄÜÓė½šŹōÄĘ·“Ó¦²śÉśĒāĘųµÄÓŠ»śĪļµÄĶ¬·ÖŅģ¹¹ĢåÓŠ_______________ÖÖ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠ±„ŗĶŅ»ŌŖ“¼µÄ»ģŗĻĪļŗĶ±„ŗĶŅ»ŌŖōČĖįµÄ»ģŗĻĪļŌŚŅ»¶ØĢõ¼žĻĀ·“Ӧɜ³É¶ąÖÖõ„µÄ»ģŗĻĪļ£¬õ„µÄĻą¶Ō·Ö×ÓÖŹĮæ·Ö±šĪŖM1”¢M2”¢M3£¬²¢ŅĄ“ĪŌö¼Ó14”£¾·ÖĪö£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖM2µÄõ„£¬ĘäÖŠŃõŌŖĖŲĖłÕ¼µÄÖŹĮæ·ÖŹżĪŖ31.4%£¬²¢ÓŠČżÖÖõ„µÄĶ¬·ÖŅģ¹¹Ģ唣
ĒėĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©Ļą¶Ō·Ö×ÓÖŹĮæĪŖM2µÄõ„µÄ·Ö×ÓŹ½ĪŖ______________________________”£
£Ø2£©±„ŗĶŅ»ŌŖōČĖįµÄ·Ö×ÓŹ½·Ö±šĪŖ______________________£¬______________________”£
£Ø3£©²Ī¼Ó·“Ó¦µÄ“¼µÄ½į¹¹¼ņŹ½ĪŖ_______________”¢_______________”¢_______________”¢_______________£Ø²»±ŲĢīĀś£©”£
£Ø4£©ÓėĻą¶Ō·Ö×ÓÖŹĮæĪŖM1µÄõ„»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬ÄÜ·¢ÉśŅų¾µ·“Ó¦£¬ÄÜÓė½šŹōÄĘ·“Ó¦²śÉśĒāĘųµÄÓŠ»śĪļµÄĶ¬·ÖŅģ¹¹ĢåÓŠ_______________ÖÖ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠ±„ŗĶŅ»ŌŖ“¼µÄ»ģŗĻĪļŗĶ±„ŗĶŅ»ŌŖōČĖįµÄ»ģŗĻĪļŌŚŅ»¶ØĢõ¼žĻĀ·“Ӧɜ³É¶ąÖÖõ„µÄ»ģŗĻĪļ£¬õ„µÄĻą¶Ō·Ö×ÓÖŹĮæ·Ö±šĪŖM1£¬M2£¬M3£¬²¢ŅĄ“ĪŌö¼Ó14”£¾·ÖĪö£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖM2µÄõ„£¬ĘäŃõŌŖĖŲĖłÕ¼µÄÖŹĮæ·ÖŹżĪŖ31.4%£¬²¢ÓŠČżÖÖõ„µÄĶ¬·ÖŅģ¹¹Ģ唣Ēė»Ų“š£ŗ
£Ø1£©Ļą¶Ō·Ö×ÓÖŹĮæĪŖM2µÄõ„µÄ»ÆѧŹ½ĪŖ ”£
£Ø2£©²Ī¼Ó·“Ó¦µÄ±„ŗĶŅ»ŌŖōČĖįµÄ»ÆѧŹ½·Ö±šĪŖ £¬ ”£
£Ø3£©²Ī¼Ó·“Ó¦µÄ“¼½į¹¹¼ņŹ½ĪŖ ”¢ ”¢ ”¢
£Ø²»±ŲĢīĀś£©”£
£Ø4£©ÓėĻą¶Ō·Ö×ÓÖŹĮæĪŖM1µÄõ„»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬ÄÜ·¢ÉśŅų¾µ·“Ó¦”¢ÄÜÓė½šŹōÄĘ·“Ó¦²śÉśĒāĘųµÄÓŠ»śĪļµÄĶ¬·ÖŅģ¹¹ĢåÓŠ ÖÖ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com