
злКЯРћгУзЊТЏУКЦј[COЃЈ60ЁЋ80%ЃЉЁЂCO2ЃЈ15ЁЋ20%ЃЉМАЮЂСПN2ЕШ]КЭСђЫсЙЄвЕЮВЦјжаЕФSO2ЃЌМШФмОЛЛЏЮВЦјЃЌгжФмЛёЕУБЃЯеЗлЃЈNa2S2O4ЃЉЃЌЦфВПЗжЙЄвеСїГЬШчЯТЃК
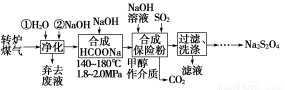
ЃЈ1ЃЉзЊТЏСЖИжЪБЃЌДцдкЗДгІЃКFe3CЃЈsЃЉЃЋCO2ЃЈgЃЉ??2COЃЈgЃЉЃЋ3FeЃЈsЃЉЃЌЦфЦНКтГЃЪ§БэДяЪНЮЊKЃН________ЁЃ
ЃЈ2ЃЉУКЦјОЛЛЏЪБЃЌЯШгУЫЎЯДдйгУNaOHШмвКЯДЕгЃЌЦфФПЕФЪЧ________ЁЃ
ЃЈ3ЃЉДгТЫвКжаЛиЪеМзДМЕФВйзїЗНЗЈЪЧ____________________________ЃЛ
ЛЙПЩЛиЪеЕФбЮРрЮяжЪЪЧ______________________________________ЃЈжЛаДвЛжжЛЏбЇЪНЃЉЁЃ
ЃЈ4ЃЉКЯГЩБЃЯеЗлЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_________________________ЁЃ
ЃЈ5ЃЉБЃЯеЗлЁЂH2O2ОљПЩгУгкжННЌЦЏАзЃЌаДГіБЃЯеЗлгыЙ§СПЕФH2O2ЃЌдкЫЎШмвКжаЗДгІЩњГЩСђЫсбЮЕШЮяжЪЕФРызгЗНГЬЪНЃК________________________________ЁЃ
ЃЈ1ЃЉc2ЃЈCOЃЉ/cЃЈCO2ЃЉЁЁЃЈ2ЃЉГ§ШЅCO2ЕШЫсадЦјЬхЁЁЃЈ3ЃЉеєСѓЁЁNa2SO3ЃЈЛђNaHSO3ЃЉ
ЃЈ4ЃЉHCOONaЃЋ2SO2ЃЋNaOH=Na2S2O4ЃЋCO2ЃЋH2O
ЃЈ5ЃЉS2O42-ЃЋ3H2O2=2SO42-ЃЋ2HЃЋЃЋ2H2O
ЁОНтЮіЁПЃЈ1ЃЉЙЬЬхЮяжЪВЛФмаДШыЦНКтГЃЪ§БэДяЪНжаЃЌKЃНc2ЃЈCOЃЉ/cЃЈCO2ЃЉЁЃЃЈ2ЃЉУКЦјжаКЌгаCO2ЕШЫсадЦјЬхЃЌЙЪгУNaOHШмвКЯДЕгвдГ§ШЅетаЉЫсадЦјЬхЁЃЃЈ3ЃЉМзДМвзШмгкЫЎЃЌЕЋЗаЕуНЯЕЭЃЌЙЪПЩгУеєСѓЗЈЛиЪеМзДМЃЛгЩгкгУЕНСЫЖўбѕЛЏСђКЭNaOHШмвКЃЌЙЪПЩЛиЪеЕФбЮРрЮяжЪЪЧNa2SO3ЛђNaHSO3ЁЃЃЈ5ЃЉБЃЯеЗлжаSЕФЛЏКЯМлЮЊЃЋ3ЃЌСђЫсбЮжаSЕФЛЏКЯМлЮЊЃЋ6ЃЌЙЪH2O2зїбѕЛЏМСЃЌРызгЗНГЬЪНЮЊS2O42-ЃЋ3H2O2=2SO42-ЃЋ2HЃЋЃЋ2H2OЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇжИЕМГхЙи Ек10СЗГЃМћгаЛњЛЏКЯЮяМАЦфгІгУСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ИЬщйжаФћУЪЯЉЕФНсЙЙПЩБэЪОЮЊ ЃЌЯТСаЙигкетжжЮяжЪЕФЫЕЗЈжае§ШЗЕФЪЧ
ЃЌЯТСаЙигкетжжЮяжЪЕФЫЕЗЈжае§ШЗЕФЪЧ
(ЁЁЁЁ)
AЃЎгыБНЕФНсЙЙЯрЫЦЃЌаджЪвВЯрЫЦ
BЃЎПЩЪЙфхЕФЫФТШЛЏЬМШмвКЭЪЩЋ
CЃЎвзЗЂЩњШЁДњЗДгІЃЌФбЗЂЩњМгГЩЗДгІ
DЃЎИУЮяжЪМЋвзШмгкЫЎ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЖўТжИДЯАжИЕМдЄВтбКЬтСЗЯАОэЃЈШ§ЃЉ ЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСаРызгдкжИЖЈЬѕМўЯТвЛЖЈФмДѓСПЙВДцЕФЪЧЃЈЁЁЁЁЃЉ
AЃЎКЌгаДѓСПOHЃЕФШмвКжаЃКCO32-ЁЂClЃЁЂFЃЁЂKЃЋ
BЃЎгыТСЗДгІВњЩњЧтЦјЕФШмвКжаЃКNaЃЋЁЂAlO2-ЁЂNO3-ЁЂHCO3-
CЃЎКЌгаДѓСПAl3ЃЋЕФШмвКжаЃКKЃЋЁЂNaЃЋЁЂNO3-ЁЂClOЃ
DЃЎЪЙМзЛљГШГЪЛЦЩЋЕФШмвКжаЃКIЃЁЂClЃЁЂNO3-ЁЂNaЃЋ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЖўТжИДЯАзЈЬтЙіЖЏСЗ4 ЛЏбЇЪЕбщСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
НќФъРДЃЌИпУЬЫсМидквћгУЫЎКЭЙЄвЕЮлЫЎДІРэСьгђЕФЯћЗбашЧѓдіГЄНЯПьЁЃЪЕбщЪвПЩгУЖўбѕЛЏУЬЮЊжївЊдСЯжЦБИИпУЬЫсМиЁЃЦфВПЗжСїГЬШчЯТЃК

ЃЈ1ЃЉЕкЂйВНжаВЩгУЬњлсліЖјВЛгУДЩлсліЕФдвђЪЧЃЈгУЛЏбЇЗНГЬЪНБэЪОЃЉ_______________________________________________________________ЁЃ
ЃЈ2ЃЉKOHЁЂKClO3КЭMnO2ЙВШлЗДгІЩњГЩФЋТЬЩЋK2MnO4ЕФЛЏбЇЗНГЬЪНЮЊ________________________________________________________________ЁЃ
ЃЈ3ЃЉЕкЂмВНЭЈШыCO2ЃЌПЩвдЪЙMnO42-ЗЂЩњЗДгІЃЌЩњГЩMnO4-КЭMnO2ЁЃдђK2MnO4ЭъШЋЗДгІЪБЃЌзЊЛЏЮЊKMnO4ЕФАйЗжТЪдМЮЊ____________________ЃЈОЋШЗЕН0.1%ЃЉЁЃ
ЃЈ4ЃЉЕкЂнВНГУШШЙ§ТЫЕФФПЕФЪЧ________________________________ЁЃ
ЃЈ5ЃЉЕкЂоВНМгШШХЈЫѕжСвКУцгаЯИаЁОЇЬхЮіГіЪБЃЌЭЃжЙМгШШЃЌРфШДНсОЇЁЂ___________ЁЂЯДЕгЁЂИЩдяЁЃИЩдяЙ§ГЬжаЃЌЮТЖШВЛвЫЙ§ИпЃЌвђЮЊ_________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЖўТжИДЯАзЈЬтЙіЖЏСЗ4 ЛЏбЇЪЕбщСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСаа№ЪіКЯРэЕФЪЧЃЈЁЁЁЁЃЉ
AЃЎгУИЩдяЕФpHЪджНМьВтЬМЫсФЦШмвКЁЂТШЫЎЁЂЯЁДзЫсЕФpH
BЃЎЭгыХЈСђЫсЗДгІЪБЃЌПЩгУеКгаХЈфхЫЎЕФУоЛЈЗХдкЕМЙмПкМьбщвнГіЕФЦјЬх
CЃЎашгУФГХЈЖШЕФNaOHШмвК450 mLЃЌдђХфжЦЪБгІбЁгУ450 mLЕФШнСПЦП
DЃЎНЋвКфх ЁЂХЈЯѕЫсБЃДцдкзиЩЋЪдМСЦПжаЃЌЗХжУгкРфАЕДІ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЖўТжИДЯАзЈЬтЙіЖЏСЗ3 дЊЫиМАЦфЛЏКЯЮяСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
гУЛЦЩЋЕФFeCl3ШмвКЗжБ№НјааЯТСаЪЕбщЃЌНтЪЭЛђНсТлВЛе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
бЁЯюЪЕбщЯжЯѓНтЪЭЛђНсТл
AМгШыFeCl3ЙЬЬхШмвКБфГЩКьКжЩЋFeCl3ЕФЫЎНтГЬЖШБфДѓ
BМгШыЕШЬхЛ§ЫЎШмвКбеЩЋБфЧГcЃЈFe3ЃЋЃЉБфаЁ
CМгШызуСПFeЗлШмвКбеЩЋБфГЩЧГТЬЩЋ2Fe3ЃЋЃЋFe=3Fe2ЃЋ
DНЋFeCl3ШмвКЮЂШШШмвКБфГЩКьКжЩЋЫЎНтЗДгІ
ІЄH>0
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЖўТжИДЯАзЈЬтЙіЖЏСЗ3 дЊЫиМАЦфЛЏКЯЮяСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЛЏбЇгыЩњВњЁЂЩњЛюУмЧаЯрЙиЁЃЯТСаа№ЪіжаЃЌВЛе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
AЃЎКНЬьЗЩЛњЩЯЕФИєШШЬеДЩЭпЪєгкИДКЯВФСЯ
BЃЎУїЗЏОЛЫЎЕФдРэКЭЁА84ЁБЯћЖОвКЯћЖОЕФдРэЯрЭЌ
CЃЎЪГДзПЩШЅГ§ЫЎЙИЃЌNH4ClШмвКПЩШЅГ§Ьњат
DЃЎSO2КЭNO2ЖМФмЪЙгъЫЎЕФpH<5.6ЃЌдьГЩЫсгъ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇЖўТжИДЯАзЈЬтЙіЖЏСЗ1 ЛЏбЇЛљБОИХФюСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
ЪГЦЗАВШЋЪЧЩчЛсЙизЂЕФШШЕуЁЃЪаГЁЩЯвбМьВтГівЛаЉПѓШЊЫЎжаКЌгаИпХЈЖШЕФжТАЉЮяЁАфхЫсбЮЁБЃЌЖјетИіЁАаавЕУиУмЁБдкШЫУЧЕФблЦЄЕзЯТБЛвўВиСЫ10ЖрФъЁЃЪЕбщЪвжажЦБИЁАфхЫсбЮЁБЙ§ГЬШчЯТЃК
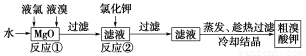
ИљОнЩЯЪізЊЛЏЙиЯЕЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЗДгІЂйЕФЛЏбЇЗНГЬЪНЮЊ______________________________ЁЃ
ЃЈ2ЃЉвбжЊЗДгІЂкЪЧИДЗжНтЗДгІЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_____________________________ЁЃ
ЃЈ3ЃЉвбжЊдкЫсадЬѕМўЯТфхЫсбЮПЩЗЂЩњШчЯТРызгЗДгІЃК
Ђё.2BrO3-ЃЋ10ClЃЃЋ12HЃЋ=5Cl2ЁќЃЋBr2ЃЋ6H2O
Ђђ.6BrO3-ЃЋ5ClЃЃЋ6HЃЋ=5ClO3-ЃЋ3Br2ЃЋ3H2O
Ђѓ.BrO3-ЃЋ5BrЃЃЋ6HЃЋ=3Br2ЃЋ3H2O
ЂйЩЯЪіЗДгІЫљЩцМАЕФСЃзгжаЃЌбѕЛЏадзюЧПЕФЪЧ________ЁЃ
ЂкдкKClКЭKBrЕФЫсадЛьКЯШмвКжаЃЌМгШыЙ§СПЕФKBrO3ЃЌЦфбѕЛЏВњЮяЮЊ________ЃЌЛЙдВњЮяЮЊ________ЁЃ
ЂлНЋ12 mL 0.4 molЁЄLЃ1 KBrO3ШмвККЭ10 mL 0.6 molЁЄLЃ1 KClШмвКдкЯЁH2SO4жаЛьКЯЃЌГфЗжЗДгІКѓЃЌВњЮяKClO3КЭCl2ЕФЮяжЪЕФСПжЎБШЮЊ________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпПМЛЏбЇвЛТжИДЯАПЮКѓбЕСЗзЈЬтСЗЯАОэЖўЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСаШмвКжаЮЂСЃЕФЮяжЪЕФСПХЈЖШЙиЯЕвЛЖЈе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
AЃЎ0.2 mol/L CH3COONaШмвККЭ0.1 mol/L HClШмвКЕШЬхЛ§ЛьКЯКѓЃКcЃЈCH3COOЃЃЉЃОcЃЈNaЃЋЃЉЃОcЃЈClЃЃЉЃОcЃЈHЃЋЃЉЃОcЃЈOHЃЃЉ
BЃЎpHЃН3ЕФбЮЫсКЭNaNO3ЕФЛьКЯШмвКжаЃКcЃЈNaЃЋЃЉЃНcЃЈClЃЃЉ
CЃЎ0.1 mol/L NaHCO3ШмвКжаЃКcЃЈNaЃЋЃЉЃЋcЃЈHЃЋЃЉЃНcЃЈHCO3-ЃЉЃЋcЃЈCO32-ЃЉЃЋcЃЈOHЃЃЉ
DЃЎЮяжЪЕФСПХЈЖШЯрЕШЕФHCNЃЈШѕЫсЃЉКЭNaCNШмвКЕШЬхЛ§ЛьКЯКѓгаЃКcЃЈHCNЃЉЃЋ2cЃЈHЃЋЃЉЃН2cЃЈOHЃЃЉЃЋcЃЈCNЃЃЉ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com