
���� A��B��C��DΪ��ѧ���������Ҿ�����ͬһ��Ԫ�أ��������и�����ת����ϵ��A�ܾ�������������C��C��ˮ��Ӧ����D��
��1����A��B��C��D��Ϊ������������ǵ�ˮ��Һ����ʹʪ�����ɫʯ����ֽ��죬���ǵ�ˮ��Һ�������ԣ���AΪH2S��BΪSO2��CΪSO3��DΪH2SO4��
��2����A��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ��������AΪNH3��D��ϡ��Һ��ʹʪ�����ɫʯ����ֽ��죬D�����ԣ���BΪNO��CΪNO2��DΪHNO3��
��3����AΪ���ʣ����Ԫ�ص�ԭ���������ӵ���ĿС��18��DΪǿ���AΪNa��BΪNa2O��CΪNa2O2��DΪNaOH���ݴ˴��⣮
��� �⣺A��B��C��DΪ��ѧ���������Ҿ�����ͬһ��Ԫ�أ��������и�����ת����ϵ��A�ܾ�������������C��C��ˮ��Ӧ����D��
��1����A��B��C��D��Ϊ������������ǵ�ˮ��Һ����ʹʪ�����ɫʯ����ֽ��죬���ǵ�ˮ��Һ�������ԣ���AΪH2S��BΪSO2��CΪSO3��DΪH2SO4��B��C�Ļ�ѧ����ʽΪ2SO2+O2$?_{��}^{����}$2SO3 ��
�ʴ�Ϊ��H2SO4��2SO2+O2$?_{��}^{����}$2SO3��
��2����A��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ��������AΪNH3��D��ϡ��Һ��ʹʪ�����ɫʯ����ֽ��죬D�����ԣ���BΪNO��CΪNO2��DΪHNO3��D��C�����ӷ���ʽΪCu+4H++2NO3-�TCu2++2NO2��+2H2O��
�ʴ�Ϊ��HNO3��Cu+4H++2NO3-�TCu2++2NO2��+2H2O��
��3����AΪ���ʣ����Ԫ�ص�ԭ���������ӵ���ĿС��18��DΪǿ���AΪNa��BΪNa2O��CΪNa2O2��DΪNaOH��C��D�Ļ�ѧ����ʽΪ2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
���� �����S��N��NaԪ�ؼ��仯����֪ʶ�������������ѧ����S��N��Na����Ԫ�ؼ��仯��������ʽ����ܽᣬ���������ǣ�ѧϰʱҪ�����ܽᣬ�γɱȽ�ϵͳ��֪ʶ�ṹ����Ŀ�ѶȲ���


 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | |
| ��ʼ����ʱ��pH | 2.7 | 7.6 | 5.2 |
| ��ȫ����ʱ��pH | 3.7 | 9.6 | 6.4 |
| ��1 | |||
| ��� | 1 | 2 | 3 | 4 | |
| �������/mL | 25.05 | 25.00 | 26.80 | 24.95 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ����NaOH��AlCl3��MgCl2���ֹ�����ɵĻ������������ˮ�У���0.58g��ɫ���������������õĻ���Һ�У�����1mol/L���ᣬ�����������������ɳ�����������ϵ��ͼ��ʾ����������NaOH����Ϊ��������
����NaOH��AlCl3��MgCl2���ֹ�����ɵĻ������������ˮ�У���0.58g��ɫ���������������õĻ���Һ�У�����1mol/L���ᣬ�����������������ɳ�����������ϵ��ͼ��ʾ����������NaOH����Ϊ��������| A�� | 3.6g | B�� | 4g | C�� | 4.4g | D�� | 4.8g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
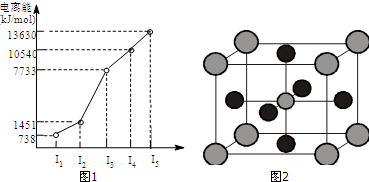
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ���ѻ� | C�� | �ѽ� | D�� | ���ѻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ϊ�Ǽ��Է��� | |
| B�� | �ڵ����г��뵪���ɷ�ֹ��˿������ | |
| C�� | ��Ԫ�ر���Ԫ�طǽ�����ǿ�������������������ǿ | |
| D�� | ����������2���Ҽ�1���м� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��������������ǣ�������
��������������ǣ�������| A�� | ���Ժ���ˮ��Ӧ | |
| B�� | �������к����ǻ����Ȼ���̼̼˫�����Ѽ� | |
| C�� | ���Է�����ȥ��ȡ����Ӧ | |
| D�� | 1 mol�������Ժ�4 mol H2�����ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com