
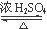 CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O 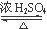 CH3COOCH2CH3 + H2OӢ
CH3COOCH2CH3 + H2OӢ

 ÄÜæ¼ŹŌĘŚÄ©³å“Ģ¾ķĻµĮŠ“š°ø
ÄÜæ¼ŹŌĘŚÄ©³å“Ģ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
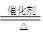 4NO+6H2O”£Ēė“ÓĻĀĶ¼ÖŠŃ”ÓĆĖłŠčµÄŅĒĘ÷(æÉÖŲø“Ź¹ÓĆ)×é³ÉŅ»Ģ×½ųŠŠøĆ·“Ó¦µÄ¼ņµ„×°ÖĆ”£ĻÖĢį¹©ŹŌ¼Į£ŗ¹żŃõ»ÆÄĘ”¢¼īŹÆ»Ņ”¢²¬·Ū”¢ĀČ»ÆøĘ”¢ÅØĮņĖį”¢ÅØ°±Ė®ŗĶĒāŃõ»ÆÄĘČÜŅŗ”£
4NO+6H2O”£Ēė“ÓĻĀĶ¼ÖŠŃ”ÓĆĖłŠčµÄŅĒĘ÷(æÉÖŲø“Ź¹ÓĆ)×é³ÉŅ»Ģ×½ųŠŠøĆ·“Ó¦µÄ¼ņµ„×°ÖĆ”£ĻÖĢį¹©ŹŌ¼Į£ŗ¹żŃõ»ÆÄĘ”¢¼īŹÆ»Ņ”¢²¬·Ū”¢ĀČ»ÆøĘ”¢ÅØĮņĖį”¢ÅØ°±Ė®ŗĶĒāŃõ»ÆÄĘČÜŅŗ”£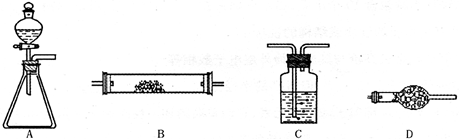
| Ń”ÓƵÄŅĒĘ÷(Ģī×ÖÄø) | ¼ÓČėµÄŹŌ¼Į | ×÷ÓĆ |
| | | |
| | | |
| | | |
| | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
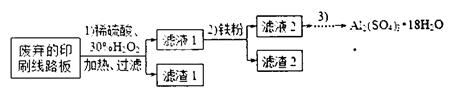
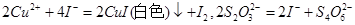
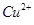 ŗ¬Į潫»į £ØĢī”°Ę«øß”±”¢”°Ę«
ŗ¬Į潫»į £ØĢī”°Ę«øß”±”¢”°Ę«
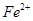 ³żČ„”£ĻĀ±ķĮŠ³öĮĖ¼øÖÖĄė×ÓÉś³ÉĒāŃõ»ÆĪļ³ĮµķµÄpH£ØæŖŹ¼³ĮµķµÄpH°“½šŹōĄė×ÓÅضČĪŖ1£®0mo1£®L-1¼ĘĖć£©”£
³żČ„”£ĻĀ±ķĮŠ³öĮĖ¼øÖÖĄė×ÓÉś³ÉĒāŃõ»ÆĪļ³ĮµķµÄpH£ØæŖŹ¼³ĮµķµÄpH°“½šŹōĄė×ÓÅضČĪŖ1£®0mo1£®L-1¼ĘĖć£©”£| | æŖŹ¼³ĮµķµÄpH | ³ĮµķĶźČ«µÄpH |
| Fe3+ | 1£®1 | 3£®2 |
| Fe2+ | 5£®8 | 8£®8 |
| A13+ | 3£®8 | 5£®2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
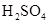 ¹²ČČÖĘČ”CO£®
¹²ČČÖĘČ”CO£®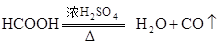
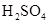 Ó¦ŌõŃł»ģŗĻ£æÅØ
Ó¦ŌõŃł»ģŗĻ£æÅØ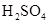 ĘšŹ²Ć“×÷ÓĆ?
ĘšŹ²Ć“×÷ÓĆ?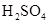 ¹²ČČÖĘČ”CO£®
¹²ČČÖĘČ”CO£®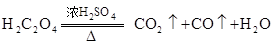
 µÄCOĘųĢå?
µÄCOĘųĢå? µÄ»¹ŌŠŌĖĒæ?
µÄ»¹ŌŠŌĖĒæ?²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
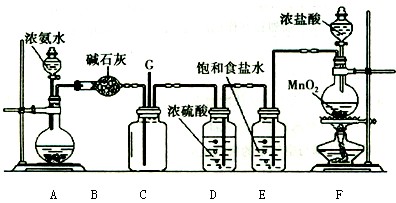
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
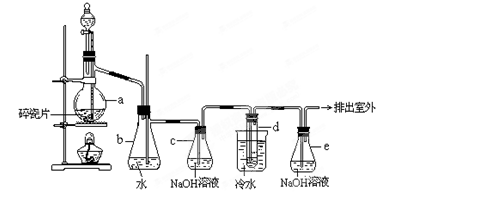
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
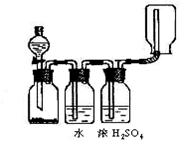
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¢Ž¢Ż¢Ü¢Ł¢Ū¢Ś | B£®¢Ž¢Ü¢Ż¢Ś¢Ł¢Ū | C£®¢Ž¢Ü¢Ł¢Ū¢Ż¢Ś | D£®¢Ś¢Ž¢Ü¢Ł¢Ū¢Ż |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com