CO��H
2�����ǵ�����������ȷ���������أ�
��1����úת��Ϊˮú����ͨ����ѧ������úת��Ϊ�ྻȼ�ϵķ���֮һ��
��֪��C��s��+O
2��g��=CO
2��g����H=-393.5kJ��mol
-1H
2��g��+
O
2��g��=H
2O��g����H=-242.0kJ��mol
-1CO��g��+
O
2��g��=CO
2��g����H=-283.0kJ��mol
-1��C��s����ˮ������Ӧ��ȡCO��H
2���Ȼ�ѧ����ʽΪ
C��s��+H2O��g��=CO��g��+H2��g����H=+131.5kJ��mol-1
C��s��+H2O��g��=CO��g��+H2��g����H=+131.5kJ��mol-1
����״���£�V�� CO����V��H
2��=1��l��ˮú��22.4L����ȫȼ������CO
2��ˮ�������ų�������Ϊ
262.5kJ
262.5kJ
��
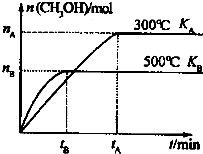
��2��һ�������£����ݻ�Ϊ3L���ܱ������з�����Ӧ��CO��g��+2H
2��g��?CH
3OH��g������ƽ��״̬������ͼʾ�ش�
��500��ʱ���ӷ�Ӧ��ʼ���ﵽƽ��״̬V��H
2��=
����n
B��t
B��ʾ��
��K
A ��K
B�Ĺ�ϵ�ǣ�K
A��
��
K
B���÷�Ӧ�ġ�H
��
��
0�����������=����������
��300���ƽ��ʱ���������ݻ�ѹ����ԭ����
�������������䣬��v������
��
��
v���棩������ڡ��������ڡ���С�ڡ�����
��3������ú��й©��������ж�������ΪCO�������������Ѫ�쵰�ף�HbO
2��������Ӧ��CO+HbO
2?O
2+HbCO��37��ʱ��K=220����[HbCO]��[HbO
2]��0.02ʱ��������CO��O
2���ʵ���Ũ��֮�ȡ�
1��11000
1��11000
ʱ���˵����������𣻰�CO�ж��IJ��˷����ѹ�����нⶾ��ԭ����
����Ũ������������ѧƽ�������ƶ���ʹCO��Ѫ�쵰�����������
����Ũ������������ѧƽ�������ƶ���ʹCO��Ѫ�쵰�����������
��




 CO��H2�����ǵ�����������ȷ���������أ�
CO��H2�����ǵ�����������ȷ���������أ�