[��ѧ-ѡ��3�����ʽṹ������]
������Ҫ�İ뵼����ϣ��������ִ����ӹ�ҵ�Ļ�������ش��������⣺
��1����̬Siԭ���У�����ռ�ݵ�����ܲ����Ϊ
M
M
�����ܲ���е�ԭ�ӹ����Ϊ
9
9
��������Ϊ
4
4
��
��2������Ҫ�Թ����Ρ�
��������
��������
�Ȼ��������ʽ�����ڵؿ��У�
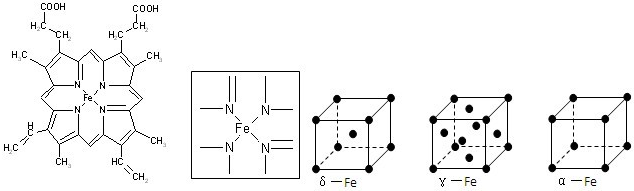
��3�����ʹ��������ʯ�ṹ���Ƶľ��壬����ԭ����ԭ��֮����
���ۼ�
���ۼ�
���ϣ��侧���й���8��ԭ�ӣ�����������λ�ù���
3
3
��ԭ�ӣ�
��4�����ʹ��ͨ�����飨SiH
4���ֽⷴӦ���Ʊ�����ҵ�ϲ���Mg
2Si��NH
4Cl��Һ�������з�Ӧ�Ƶ�SiH
4���÷�Ӧ�Ļ�ѧ����ʽΪ
Mg2Si+4NH4Cl=SiH4+4NH3+2MgCl2
Mg2Si+4NH4Cl=SiH4+4NH3+2MgCl2
��
��5��̼����йػ�ѧ������������ʾ����Ҫ�����ͽ��������й���ʵ��
| ��ѧ�� |
C-C |
C-H |
C-O |
Si-Si |
Si-H |
Si-O |
| ����/��kJ?mol-1�� |
356 |
413 |
336 |
226 |
318 |
452 |
�ٹ���̼ͬ�壬Ҳ��ϵ���⻯�������������������϶�Զ���������࣬ԭ����
C-C����C-H����ǿ�����γɵ������ȶ�����������Si-Si����Si-H���ļ��ܽϵͣ����ѣ����³��������������ɣ�
C-C����C-H����ǿ�����γɵ������ȶ�����������Si-Si����Si-H���ļ��ܽϵͣ����ѣ����³��������������ɣ�
��
��SiH
4���ȶ���С��CH
4���������������ԭ����
C-H���ļ��ܴ���C-O����C-H����C-O���ȶ�����Si-H���ļ���ȴԶС��Si-O��������Si-H�����ȶ����������γ��ȶ��Ը�ǿ��Si-O��
C-H���ļ��ܴ���C-O����C-H����C-O���ȶ�����Si-H���ļ���ȴԶС��Si-O��������Si-H�����ȶ����������γ��ȶ��Ը�ǿ��Si-O��
��
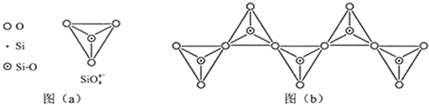
��6���ڹ������У�SiO
�����壨����ͼ��a����ͨ�����ö��������ӿ��γɵ�״����״����״���Ǽ���״�Ĵ���ṹ��ʽ��ͼ��b��Ϊһ�����������ṹ�Ķ�����������Siԭ�ӵ��ӻ���ʽΪ
sp3
sp3
��Si��O��ԭ����֮��Ϊ
1��3
1��3
����ѧʽΪ
SiO32-
SiO32-
��



 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�

 ��2012?������ģ��[��ѧ--ѡ��3�����ʽṹ������]�飨As����һ����Ҫ�Ļ�ѧԪ�أ�����γɶ�����;�㷺�Ļ����
��2012?������ģ��[��ѧ--ѡ��3�����ʽṹ������]�飨As����һ����Ҫ�Ļ�ѧԪ�أ�����γɶ�����;�㷺�Ļ���� ����ѧ--ѡ��3�����ʽṹ�����ʡ�
����ѧ--ѡ��3�����ʽṹ�����ʡ�