
��1������������Ϊ28%��KOHˮ��Һ�У�OH-������H2O������֮����_______��
��2����KCl��CaCl2����ɵ�ij������У�K+��Ca2+�����ʵ���֮��Ϊ2��1����û�����к�CaCl2����������Ϊ__________��KCl��CaCl2�����ʵ���֮��Ϊ__________����1 mol Cl-�ĸû�����������____________g��
��3���ڱ�״���£���CO��CO2��ɵĻ������13.44 L,����Ϊ20 g,�û�������У�̼��������ԭ�ӵ���Ŀ֮��Ϊ__________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
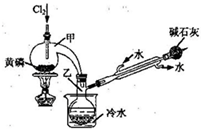 ��2011?�Ͼ���ģ��PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
��2011?�Ͼ���ģ��PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�| �۵�/�� | �е�/���� | �ܶ�/g?cm-3 | ���� | |||||||||
| ���� | 44.1 | 280.5 | 1.82 | 2P��������+3Cl2
| ||||||||
| PCl3 | -112 | 75.5 | 1.574 | ��ˮ����H3PO3��HCl����O2����POCl3 | ||||||||
| POCl3 | 2 | 105 3 | 1.675 | ��ˮ����H3PO4��HCl��������PCl3 |
2.75(c1v1-
| ||
| m |
2.75(c1v1-
| ||
| m |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2����KCl��CaCl2����ɵ�ij������У�K+��Ca2+�����ʵ���֮��Ϊ2��1����û�����к�CaCl2����������Ϊ__________��KCl��CaCl2�����ʵ���֮��Ϊ__________����1 mol Cl-�ĸû�����������____________g��
��3���ڱ�״���£���CO��CO2��ɵĻ������13.44 L,����Ϊ20 g,�û�������У�̼��������ԭ�ӵ���Ŀ֮��Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com