
ОӘСйЦӨҪНДёҫъДЬФЪСхідЧгКұҪшРРУРСхәфОьЈ¬УЦДЬФЪИұСхКұҪшРРОЮСхәфОьЈЁТ»°гАҙЛөЈ¬ТТҙј·ўҪНКЗәвБҝЛьөДТ»ёцұкЧјЈ©ЎЈЧцБЛИзПВКөСйЈәөЪТ»Жҝҫӯ№эГрҫъөДЖПМСМЗИЬТәідИлСхЈ¬ҪУИлҪНДёҫъҫъЦЦҪшРРЕаСшЈ¬Ів¶ЁЖдөҘО»КұјдДЪөДОьКХСхөДОпЦКөДБҝәНІъЙъ¶юСх»ҜМјөДОпЦКөДБҝЎЈҫЭҙЛ»ШҙрУлХвёцКөСйУР№ШөДОКМвЈ¬·ЦұрҙУЛщБРСЎПоЦРСЎіцЛьГЗөДХэИ·ҙр°ёЎЈ
ЈЁ1Ј©ФЪҪНДёҫъЦ»ҪшРРУРСхәфОьКұЈ¬КФұИҪПОьКХСхөДОпЦКөДБҝәНІъЙъ¶юСх»ҜМјөДОпЦКөДБҝөДҙуРЎ
[ЎЎЎЎ]
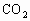 ІъЙъБҝҙу
ІъЙъБҝҙуЈЁ2Ј©ИфҪНДёҫъН¬КұҪшРРУРСхәфОьәНТТҙј·ўҪНЈ¬јЩИзФЪУРСхәфОьәНТТҙј·ўҪН№эіМЦРУРөИБҝөДЖПМСМЗұ»ПыәДөфЈ¬ХвКұОьКХСхөДОпЦКөДБҝУлІъЙъ¶юСх»ҜМјөДОпЦКөДБҝЦ®ұИКЗ
[ЎЎЎЎ]
ЈЁ3Ј©ИфҪНДёҫъН¬КұҪшРРУРСхәфОьәНТТҙј·ўҪНЈ¬Из№ыУРСхәфОьәНТТҙј·ўҪНЛщІъЙъ¶юСх»ҜМјөДОпЦКөДБҝПаөИЈ¬ХвКұОьКХСхөДОпЦКөДБҝУлІъЙъ¶юСх»ҜМјөДОпЦКөДБҝЦ®ұИКЗ
[ЎЎЎЎ]
ЈЁ4Ј©ИфјмІвөДҪб№ыКЗОьКХСхөДОпЦКөДБҝУлІъЙъ¶юСх»ҜМјөДОпЦКөДБҝЦ®ұИОӘ3ЎГ5Ј¬ЖдФӯТтКЗ
[ЎЎЎЎ]
AЈ®УР1ЈҜ4өДҪНДёҫъФЪҪшРРУРСхәфОь
BЈ®УР1ЈҜ3өДҪНДёҫъФЪҪшРРУРСхәфОь
CЈ®УР1ЈҜ2өДҪНДёҫъФЪҪшРРУРСхәфОь
DЈ®УР2ЈҜ3өДҪНДёҫъФЪҪшРРУРСхәфОь
ЈЁ5Ј©ИфОЮСхәфОьПыәДБЛ360 mgөДЖПМСМЗЈ¬ҝЙЙъіЙЈЁЎЎЎЎЈ©өДТТҙјЎЈ
[ЎЎЎЎ]
ЈЁ6Ј©УлҪНДёҫъПаұИЈ¬ҙЧЛбҫъПё°ыҪб№№ЙПөДЦчТӘІоұрКЗ________ЎЈ
|
ЈЁ 1Ј©CЈЁ2Ј©DЈЁ3Ј©BЈЁ4Ј©BЈЁ5Ј©AЈЁ6Ј©ОЮіЙРОөДПё°ыәЛЈ¬јҙОЮәЛДЈҪб№№Ј¬іэәЛМЗМеНвОЮЖдЛыПё°ыЖчөгІҰЈәЈЁ 1Ј©ҪНДёҫъҪшРРУРСхәфОьКұЈ¬Жд·ҙУҰКҪКЗ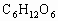 Ј«6 Ј«6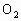  6 6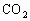 Ј«6 Ј«6 Ј«ДЬБҝЈ¬ҙУ·ҙУҰКҪҝЙТФҝҙіцҪНДёҫъҪшРРУРСхәфОьКұОьКХСхЖшөДДҰ¶ыКэәН·Еіц Ј«ДЬБҝЈ¬ҙУ·ҙУҰКҪҝЙТФҝҙіцҪНДёҫъҪшРРУРСхәфОьКұОьКХСхЖшөДДҰ¶ыКэәН·Еіц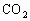 өДДҰ¶ыКэПаөИЎЈЈЁ2Ј©ЈЁ3Ј©ЈЁ4Ј©ЈЁ5Ј©ОКТӘАыУГҪНДёҫъУРСхәфОьәНОЮСхәфОь·ҙУҰКҪҪвМвЎЈ өДДҰ¶ыКэПаөИЎЈЈЁ2Ј©ЈЁ3Ј©ЈЁ4Ј©ЈЁ5Ј©ОКТӘАыУГҪНДёҫъУРСхәфОьәНОЮСхәфОь·ҙУҰКҪҪвМвЎЈ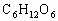  2 2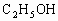 Ј«2 Ј«2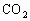 Ј«ДЬБҝЈ¬ Ј«ДЬБҝЈ¬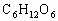 Ј«6 Ј«6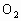  6 6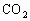 Ј«6 Ј«6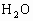 Ј«ДЬБҝЎЈ Ј«ДЬБҝЎЈ
ИфҪНДёҫъУРСхәфОьәНТТҙј·ўҪНПыәДөИБҝөДЖПМСМЗЈ¬ФтПыәД 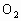 өДБҝУл·Еіц өДБҝУл·Еіц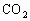 өДБҝөДұИОӘ6ЎГЈЁ6Ј«2Ј©ЈҪ3ЎГ4ЎЈИфУРСхәфОьәНТТҙј·ўҪНЛщІъЙъөД өДБҝөДұИОӘ6ЎГЈЁ6Ј«2Ј©ЈҪ3ЎГ4ЎЈИфУРСхәфОьәНТТҙј·ўҪНЛщІъЙъөД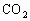 өДОпЦКөДБҝПаөИЈ¬ФтОьКХСхөДОпЦКөДБҝУлІъЙъ өДОпЦКөДБҝПаөИЈ¬ФтОьКХСхөДОпЦКөДБҝУлІъЙъ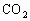 өДОпЦКөДБҝЦ®ұИОӘ1ЎГ2Ј¬ТтОӘУРСхәфОьПыәДөД өДОпЦКөДБҝЦ®ұИОӘ1ЎГ2Ј¬ТтОӘУРСхәфОьПыәДөД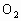 БҝәН·Еіц БҝәН·Еіц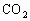 өДБҝПаөИЎЈ өДБҝПаөИЎЈ
ИфјмІвөДҪб№ыКЗОьКХСхөДБҝУлІъЙъ 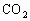 өДБҝЦ®ұИОӘ3ЎГ5Ј¬ФтЙиҪшРРУРСхәфОьөДҪНДёҫъХјxЈ¬ФтҪшРРОЮСхәфОьөДҪНДёҫъОӘ1ЈӯxЈ¬АыУГТФЙПБҪ·ҙУҰКҪөГИзПВ·ҪіМКҪ6xЈә[6xЈ«2ЈЁ1ЈӯxЈ©]ЈҪ3ЎГ5Ј¬ҪвөГxЈҪ өДБҝЦ®ұИОӘ3ЎГ5Ј¬ФтЙиҪшРРУРСхәфОьөДҪНДёҫъХјxЈ¬ФтҪшРРОЮСхәфОьөДҪНДёҫъОӘ1ЈӯxЈ¬АыУГТФЙПБҪ·ҙУҰКҪөГИзПВ·ҪіМКҪ6xЈә[6xЈ«2ЈЁ1ЈӯxЈ©]ЈҪ3ЎГ5Ј¬ҪвөГxЈҪ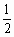 ЎЈ ЎЈ
ЈЁ 6Ј©ҪНДёҫъКЗХжәЛЙъОпЈ¬УРіЙРОөДПё°ыәЛЈЁХжХэөДПё°ыәЛЈ©Ј»ҙЧЛбҫъКЗПёҫъКЗФӯәЛЙъОпЈ¬ОЮіЙРОөДПё°ыәЛЈЁХжХэөДПё°ыәЛЈ©ЎЈ |


 ФД¶БҝміөПөБРҙр°ё
ФД¶БҝміөПөБРҙр°ё
| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
°Щ¶ИЦВРЕ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com