

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��2012��ȫ����ͨ�ߵ�ѧУ����ͳһ�����������ﲿ�֣���ٰ�ȫ������������ ���ͣ��ۺ���
(8��)ijͬѧΪ��̽��pH������Һ����ø���Ե�Ӱ�죬�������ʵ�鲽�裺
����A��B��C��D��E5֧�Թ��зֱ����pH5.0��6.0��7.0��8.0��9.0������Ũ�Ȼ���Һ5mL���ٷֱ������������Ϊ1%�ĵ���Һ1mL��
�ڸ��Թ��зֱ�����ʵ�Ũ�ȵ���Һϡ��Һ1mL��ҡ�ȡ�
�۽�5֧�Թܷ���70�����ˮԡ�У�����ʱ����ͬ�Һ��ʡ�
��ȡ�����Թܣ��ֱ��������Լ�2mL��ҡ�ȡ�
�ݹ۲���Թ���Һ����ɫ��ͨ����ɫ��dz�ж���Һ����ø���õ�����pH
����ʵ�鲽������2�������������˵�����������ɣ��������Լ���Ũ�Ⱥͼ�������pH�ݶ��Լ�ʵ����ظ����������Ա�ʵ���ܵõ���ȷ��Ԥ�ڽ����
��1�� ��
��2�� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2014��㶫տ����ʮһ�и�����һ���¿�������������棩 ���ͣ��ۺ���
��12�֣�����������Ϊ�о���ˮʱ KNO3 ����ӣ�Ҹ�������Ӱ�죬������������ӣ�ң�����һ��������ˮ����������ֱ����벻ͬŨ�ȵ� KNO3 ��Һ������Һ��߳��������棬ÿ�춨ʱ�ⶨ��ӣ�Ҹ������������ʣ������ͼ��
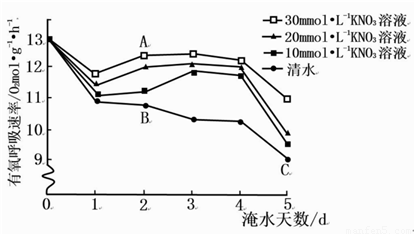
��ش�
ϸ�������������� CO2 �ij����� ������ͼ�� A��B��C ���㣬��֪ ���ڵ�λʱ����������ϵ�[H]��ࡣ
ͼ�н����ʾ����ˮʱ KNO3 ����ӣ�Ҹ������������ʽ����� ���ã����� mmol��L-1 �� KNO3 ��Һ����Ч����á�
��ˮȱ��ʹ���ϲ��ֺ�ϵ���������ܵ��谭�����ϲ���Ҷɫ��ƣ�Ҷ���غ������٣�ʹ�ⷴӦΪ����Ӧ�ṩ��[H]�� ���٣���ϵȱ���ᵼ�¸�ϸ������������ǿ��ʵ��������ܷ���� CO2 ��Ϊ��������������ʵ�ָ�ꣿ �����˵�� ��
��.��6�֣�ijͬѧΪ��̽��pH������Һ����ø���Ե�Ӱ�죬���������ʵ�鲽�裺
����A��B��C��D��E5֧ʹ���ڷֱ����pH5.0��6.0��7.0��8.0��9.0������Ũ�Ȼ���Һ5ml���ٷֱ������������Ϊ1%�ĵ���Һ1ml��
�ڸ��Թ��зֱ�����ʵ�Ũ�ȵ���Һϡ��Һ1ml��ҡ�ȡ�
�۽�5֧�Թܷ���70�����ˮԡ�У�����ʱ����ͬ�Һ��ʡ�
��ȡ�����Թܣ��ֱ��������Լ�2ml��ҡ�ȡ�
�ݹ۲���Թ���Һ����ɫ��ͨ����ɫ��dz�ж���Һ����ø���õ�����pH��
����ʵ�鲽������2�������������˵�����������ɣ��������Լ���Ũ�Ⱥͼ�������pH�ݶ��Լ�ʵ���ظ����������Ա�ʵ���ܵõ���ȷ��Ԥ�ڽ����
��1�� ����2�� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2014�캣�������λ���ѧ�߶���ѧ�ڽ�ѧ������������Ծ��������棩 ���ͣ�ѡ����
����ø�ܹ��ֽ�������������ˮ���ij�֭�ʡ�ijͬѧΪ��̽��pH�Թ���ø���Ե�Ӱ�죬�ڲ�ͬpH�£��������Ĺ���ø���뵽������ƻ�����У��ڷ�Ӧͬ��ʱ����ٽ���ӦҺ����ͬ��ʱ�䣬����Ͳ����˳�ƻ��֭���������������ȷ��ӳʵ������������(����)
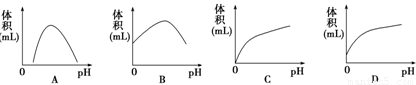
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2012��ȫ����ͨ�ߵ�ѧУ����ͳһ�����������ﲿ�֣���ٰ�ȫ���������棩 ���ͣ��ۺ���
(8��)ijͬѧΪ��̽��pH������Һ����ø���Ե�Ӱ�죬�������ʵ�鲽�裺
����A��B��C��D��E5֧�Թ��зֱ����pH5.0��6.0��7.0��8.0��9.0������Ũ�Ȼ���Һ5mL���ٷֱ������������Ϊ1%�ĵ���Һ1mL��
�ڸ��Թ��зֱ�����ʵ�Ũ�ȵ���Һϡ��Һ1mL��ҡ�ȡ�
�۽�5֧�Թܷ���70�����ˮԡ�У�����ʱ����ͬ�Һ��ʡ�
��ȡ�����Թܣ��ֱ��������Լ�2mL��ҡ�ȡ�
�ݹ۲���Թ���Һ����ɫ��ͨ����ɫ��dz�ж���Һ����ø���õ�����pH
����ʵ�鲽������2�������������˵�����������ɣ��������Լ���Ũ�Ⱥͼ�������pH�ݶ��Լ�ʵ����ظ����������Ա�ʵ���ܵõ���ȷ��Ԥ�ڽ����
��1�� ��
��2�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com