
��9�֣������Ŵ����������������ߣ�����Ŵ�ѧ�о����ܹ�ע�������о�����ijЩˮ���緬�ѵ������ɷֶԲ���֢״�л������á�����������Ϣ�ش����⣺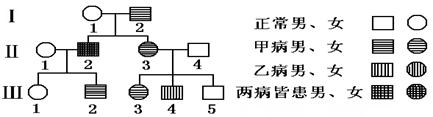
��1����ͼΪ�����Ŵ���ϵ��ͼ���ײ�������A�����ʾ���Ҳ�������B��b��ʾ����-4���²����ײ����Ŵ���ʽΪ____________���Ҳ����Ŵ���ʽΪ____________��
��2����-2�Ļ�����Ϊ____________�������-2���-3���䣬�����������ӵĸ���Ϊ____________��
����ֲ��������ë��A��a����ʵ����ɫ��B��b����λ�����Գ�Ⱦɫ���ϵĵ�λ������ơ���֪����ë���Ŵ��У�ij�ִ��ϻ����͵ĺ��Ӿ�������ЧӦ����������ߵķ��������������������������ӽ�ʵ�飺
ʵ��1������ë���������ë���������ë���:����ë���=2:1
ʵ��2������ë���������ë���������ë���:����ë�ƹ�:����ë���:����ë�ƹ�=3:1:3:1
ʵ��3������ֲ����ҷ���ֲ�������ë���:����ë�ƹ�:����ë���:����ë�ƹ�=2:2:1:1
��1������ֲ���У��������ӵĻ�������______________������һ��ȷ��ʵ��1�Ӵ��е�����ë������ѵĻ�����������ķ�����_____________________________________����2�֣�
��2��ʵ��3���ױ��ı����ͷֱ���______________��______________��
��1����Ⱦɫ�������Ŵ� ��X�����Ŵ�
(2)AaXbY 7/32
��(1)AA ����ȫ���Խ������ݺ����ʵ����ɫ�������ж�
(2)����ë��� ����ë�ƹ�
���������������1����ͼΪ�����Ŵ���ϵ��ͼ���ɢ�-3�����Ҳ����͢�-4�����Ҳ����õ���-3�����Ҳ���������������Ϊ���ԡ�����֪�Ҳ��������Ŵ�������-4���²�����ͬʱֻ�����Ի����������Ҳ����Ŵ���ʽΪ��X�����Ŵ����ײ���I-2����-3����-3���������֣����Լײ����Ŵ���ʽΪ��Ⱦɫ�������Ŵ���
��2����-2�Ļ�����ΪAaXbY�������-2��A��XBY�����-3��A��XBXB��A��XBXb�����䣬�����������ӵĸ���Ϊ1/4��1-1/2��1/4��=7/32��
��1������ʵ��1������ë���������ë���������ë���:����ë���=2��1���Ʋ�����ë����Ļ�������AaBB��ͬʱ����ֲ���У��������ӵĻ�������AA������һ��ȷ��ʵ��1�Ӵ��е�����ë������ѵĻ�����������ķ���������ȫ���Խ������ݺ����ʵ����ɫ�������жϡ�
����ʵ��2������ë���������ë���������ë���������ë�ƹ�������ë���������ë�ƹ�=3��1��3��1���Ʋ�����ë����Ļ�������AaBb������ë����Ļ�������aaBb��
����ʵ��3������ֲ����ҷ���ֲ�������ë���������ë�ƹ�������ë���������ë�ƹ�=2��2��1��1�����Էֽ�����ף�����ë=2��1�������������ӵĻ�������AA�������ױ���Aa��������ƹ�=1��1�����ԣ������ױ���Bb��bb������ױ���������AaBb��Aabb��������������ë���������ë�ƹ���
���㣺���⿼���Ŵ����ͻ���������϶��ɵ�������������ڿ��鿼��������ѧ֪ʶ��Ҫ�㣬����֪ʶ���������ϵ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��14�֣�
��8�֣�����Ϊ�۲�DNA��RNA��ϸ���зֲ���ʵ�飬�����ʵ��ش����⣺
�����þߣ���У����̡�������ȾҺ����������Ϊ8�������ᡣ��Ƭ���Dz�Ƭ�����ӣ������룬���ܣ�����ˮ����ˮֽ�������ȡ�


 �������裺��ȡ�����ƬҶ�ڱ�Ƥϸ�� ˮ�� Ưϴ Ⱦɫ
�������裺��ȡ�����ƬҶ�ڱ�Ƥϸ�� ˮ�� Ưϴ Ⱦɫ ��Ƭ �۲�
��Ƭ �۲�
��1���۲�Ľ���ǣ���____________ɫ������Ҫ�ֲ���ϸ�����У���___________ɫ������Ҫ�ֲ���ϸ�����С�
��2��������ÿ�ǻ��Ƥϸ��������Ҫ����ǻ��Ƥϸ��������������Ϊ0.9����NaCl��Һ�е�Ŀ���ǣ�____________
��3������������Ϊ8��������ˮ���Ŀ���ǣ�1.________________2._________________��
��4��������ˮƯϴ��Ŀ���ǣ�
��5��������ϸ����Я��_____________�����ʣ����������Ŵ��������_______������ϳ��о�����Ҫ���á�
��6�֣���ͼ��ijͬѧ�ڡ�Ŀ��10�����ᄉ40����ʱ�����¿�����ͼ��ͼ�ش�
��1������ѡ�����й�������ʹ�ò���ģ�ǰһ���Dz�������һ����Ŀ�ģ����д������____
A��ת��ת���������ò�ͬ�Ŵ������ᄉ
B������ϸ�������������ᄉ�벣Ƭ�걾֮��ľ���
C�����ڹ�Ȧ��������Ұ�Ĵ�С
D�����ڷ��⾵��������Ұ������
��2������ͼ�Т���ָ��ϸ���Ƶ���Ұ��������й۲죬��װƬӦ��________���ƶ���
��3����װƬ�ϵ�ϸ���ɵ�����ȷֲ�����ô��ͬѧ��Ŀ�����������£�����10�����ᄉȥ�۲���ͬ��λ��ϸ���������ۼ��㣬��Ұ�ڿ����ɵ�����ϸ����ĿΪ________����
��4���۲첣Ƭ�걾ʱ����������Ұ���ϰ����Ҳ��������Ӧ����________��
A��Ŀ�� B���ᄉ C����Ȧ D�����⾵
��5����һ�ܹ�ѧ�����ľ�������4����ͷ���ס���һ�������ƣ��ϳ����ҽ϶̣������������ƣ����ϳ������϶̡���Ŵ������ľ�ͷ�����________��(2��)
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��5�֣���ͼ�ǹ���̽����ĸ��ϸ��������ʽ������ʵ��װ�ã����ͼ�ش����⣺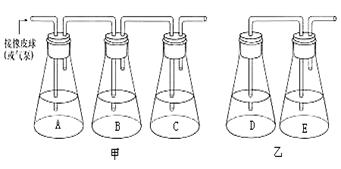
��1���ֱ���B.Dƿ�м����ĸ���������ʯ��ˮӦ�ӵ� ƿ�У�Aƿ������NaOH��Һ�������� ��
��2��һ��ʱ�����B.Dƿ��ȡ��������Һ���μ� ��Һ���ƾ��Ĵ��ڣ���ɫ�仯Ϊ ��
��3��д����װ���з������õ��ܷ�Ӧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��8�֣���ͼ�Ǽף�����ΪA��a�����ң�����ΪB��b�����ֵ������Ŵ������Ŵ�ϵ��ͼ�����Т�6Я���ײ�������֪�������о���غ�跢�������YȾɫ��Ƭ���ƽӵ�����Ⱦɫ���Ϻ���Ⱥ�л������Ⱦɫ��ΪXX�����ԣ����4��������ش�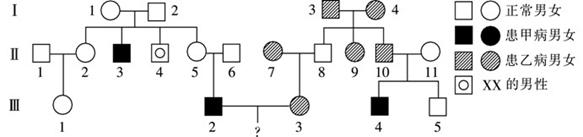
��1���ײ����Ŵ���ʽ�� ����5�͢�6����һ�������к��ĸ���Ϊ ��
��2���Ҳ����Ŵ���ʽ�� ����10�Ļ�����Ϊ ��
��3������3���мײ��²��������2�͢�3��飬��һ���������ӵĸ���Ϊ ����һ��ֻ��һ�ֲ����ӵĸ���Ϊ ��
��4����4����ı������� ������1Ϊ����ɫä����Я���ߣ���2���������4������ɫä�ĸ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��10�֣��о���������ϸ���������ֻ�ϸ�������������²�����ATP����û�����Բ��죬����ϸ�����ڻ�������ȡ������ϸ��������������������ϸ�������ɱ�����ͼ�ǰ�ϸ�������������������ǵIJ��ִ�л���̣���ͼ�����ش����⣺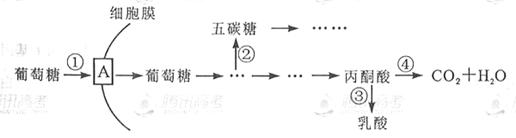
��1��ͼ��A����ϸ��Ĥ�ϵ� �������ǽ��방ϸ�����ڴ�л�����п�
ͨ���γ���̼�Ƕ��ϳ� ��ΪDNA���Ƶ�ԭ�ϡ�
��2�������������£���ϸ���������õķ�ʽΪ ����������ϸ����Ȣ١��ܹ����ڰ�ϸ����������ǿ���� �����ţ���
��3����Ҫ����ҩ�������ư�֢����ϸ���е��쳣��л;����ͼ�й��� �����ţ�����ѡΪ����λ�㡣
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
(6��)������ԱΪ̽���յ���ĸԭ��������ȡ�϶�Ҷ�������������������������ʵ�顣
�� ��ȡ�϶�Ҷ���壺���˲϶�����ҶƬ��ĥҺ������Һ����_A_����Ҷ���壬����2 mL 0.35 mol��L��1�Ȼ�����Һ���Ƴ�����Һ��
�� �Ʊ���ĸԭ�����壺��������������2%����ţø������ĸ������ý�ĸԭ�����塣
�� �յ���ĸԭ��������ȡ�϶�Ҷ���壺����ĸԭ��������϶�Ҷ�����ͺ��ڲ�ͬ�¶Ⱥ�pH���þ��Ҷ���(PEG)����60 min��ϴ�ӡ��������۲죬������±���
| ʵ����� | �¶� | pH | ��ĸԭ��������/mL | ��Ҷ����Ľ�ĸԭ��������/mL |
| 1 | 30�� | 7.6 | 760 | 4 |
| 2 | 37�� | 7.6 | 1 100 | 7 |
| 3 | 37�� | 6.0 | 1 250 | 5 |
| 4 | 37�� | 10.2 | 990 | 3 |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��8�֣�Ϊ̽����Ũ��NaHSO3��Һˮ��������ʵ�Ӱ�죬ij�о�С����������ʵ�飬�����ʵ�鱨�沢�ش��������⣺
��1��ʵ�鱨��
�ٲ����þߣ������ڵ��������ԣ�1mmol/L NaHSO3��Һ������ˮ���������Ϸ����ⶨ�ǵȡ�
��ʵ�鲽�裺
��һ����ѡȡ�������͡����ơ�ҶƬ��һ��ˮ��ֲ�����ƽ���ֳɼס������飬����Ϊ�����飬����Ϊʵ���顣
�ڶ�����ÿ������ֱ����� �� �������ڼס��������ˮ��ҶƬ�ϣ���������ⶨ������ʡ�
�۽���������ʵ������ͼ1�����������Եó��Ľ����� ��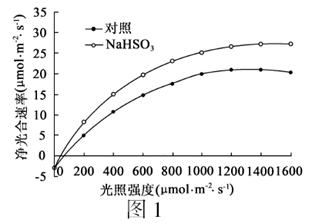
��2������NaHSO3������Ҷ�����γ�ATP���������Ʋ������ò�λ��Ҷ����� ��
��3���о�С�����������������ʵ�飬֤����0.2µmol/L���������ຣ�PMS����1mol/LNaHSO3ЧӦ��ͬ�����߹�ͬ�����Ȳ������ۼ�ЧӦ��Ҳ������ЧӦ�����ڴ����ָ��λ��������ͼ���Ƴ�ʵ������ʵ�����ǿ��Ϊ1000µmol?m-2?s-1������ 4�֣�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��9�֣�������ΪAaBb�����Ի���������ϣ��Ķ�����ˮ��������ϸ���к���24��Ⱦɫ�塣�����Ļ�ҩ���������������ڻ���ɻ��۷����ɵĵ�����ֲ���ͬʱ��Ҳ��õ�һЩ�ɻ�ҩ��ϸ�������ɵĶ�����ֲ�ꡣ
(1)�ɻ��۷����ɵĵ�����ֲ����ɻ�ҩ��ϸ�������ɵĶ�����ֲ���������ڣ���̬�ϲ������Բ�𣬿��Բ��������۲�ķ����������Ǽ������֡�
д���������۲���װƬ�Ļ�������____ ________________��
(2)�Ի�ҩ�������������еõ���δ���ֵ����ߣ���0.2%һ0.4%����ˮ���ؽ��д������õ�������Ⱦɫ����Ŀ����һ����ֲ�ꡣ��ͼ�Ǹù����� ϸ�κ�DNA�����ı仯ʾ��ͼ��
�ٲ�����ͻ�䣬��h~i�����У������� ____ ��Ⱦɫ���飬��Ⱦɫ����Ļ������ ____ ���һ����������һ��������ͬ��
�ڻ�ҩ�е�������Ⱦɫ����Ŀ���룬����δʧȥȫ���ԣ�ԭ���� ____ ����ˮ������ ____ ����ͼ����ĸ���𣩹����з����յ�Ⱦɫ��ӱ������á�
����32P��ǵ�����ˮ������ϸ����DNA����˫�����ٷ��벻��32P�����������������ڵڶ���ϸ�����ѵĺ��ڣ�ϸ���б���ǵ�Ⱦɫ���� ____ ��������ϸ��ijһ����Ⱦɫ������ ________ ������ǡ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��7�֣�ijУ������ȤС����ѧϰ�˿α�ʵ�顰̽����ĸ����ϸ��������ʽ�������һ��̽����ĸ���������������������²�������CO2ʱ���������������������ǽ϶�����⡣���ǽ���������ʵ�飺��һ��Ũ�ȵ������ȴ����������Һ��������ĸ�����Ⱥ���ͼװ��ʵ�飨ʵ��ǰƿ�о��������������ⶨ���ס���װ����CaCO3�������ʱ����ȥװ�ã����ס�������ƿ��Һ�ֱ����˾�Ĥ���ˣ���ȥ��ĸ�����õ���Һ1����Һ2��������ش�
��1���ס���������Ա����� ��ʵ�����轫����Һ��������ҺŨ�ȵȿ�������ͬ�����ˣ���Щ������ ������
��2����ĸ���ܲ���CO2�ij����� ����Ӧ������CO2��������������ݷ���ˮ��Һ��⣬����ɫ�仯�� ��
��3���������̽��ʵ�飬���� �Լ����� �����£�����Һ1����Һ2���м�⣬�Ƚ�������Һ�� �����ж���ʣ�������ǵĶ��١�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com