
��1�����䷽������ֲ������Ĵ���Ԫ����____________________________����Ԫ����________________________________��
|
�ɷ� |
���� |
|
Ca(NO3)2 |
1g |
|
KH2PO4 |
0.25g |
|
KCl |
0.12g |
|
MgSO4 |
0.25g |
|
Fe(NO3)2 |
0.25g |
|
�����Ԫ�� |
������ |
|
����ˮ |
1000ml |
��2�����������ڣ�ֲ�����ή����������ܵ�ԭ����________���ɲ�ȡ�������Ч�Ĵ�ʩ��________��
��3������ȥ����Һ��MgSO4����ֱ��Ӱ��ֲ������________�ĺϳɣ�����ȥCa(NO3)2������________�ĺϳ����裬ֲ�곤�ư�С��
��4��Ҫʹ��ˮ��ֲ�������������Һ������Ӧ����________�ֱ���Ԫ�ء��������Ĺ����о���������Һͨ���������Ŀ����________�����ڿ���Ԫ�ص����ա�
��5��������һ��ʱ��������Һ�������![]() ���ٶ�Ca2+�����ӽ϶࣬��һ������ϸ��Ĥ��________�йء�
���ٶ�Ca2+�����ӽ϶࣬��һ������ϸ��Ĥ��________�йء�
��6��PԪ����ֲ����ı������Ԫ�أ����빹��������Ҫ��������о����ֺ�PԪ�صĻ������˵���û�������ֲ��������������ã�
�����________���ã�________________________________________
�����________���ã�________________________________________
�����________���ã�________________________________________
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��3+x�߿��߷־������� ���ͣ�043
�±�Ϊ��������ʱ���õ�һ������Һ���䷽���Ը��ݱ������ݣ�������ѧ֪ʶ�����ش��������⣮
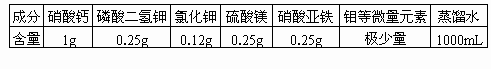
(1)������Һ��������________Ԫ�ز��ǿ���Ԫ�أ������Ŀ���Ԫ���У�________���ڲ��ܱ�ֲ���ٶ����õĴ���Ԫ�أ�
(2)Ҫ������������������ˮ�������Һ������Ϊ����Ԫ����________��ʽ��ֲ��ϸ�����գ�
(3)����Һ��Ũ�ȱ�ϸ��Һ��Ũ�ȵͣ�����Ա�ֲ֤��ϸ��������Һ������________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�071
�±�Ϊ��������ʱ���õ�����Һ�䷽���Ը��ݱ��ش�
|
�ɷ� |
����� |
�������� |
�Ȼ��� |
����þ |
�������� |
�����Ԫ�� |
����ˮ |
|
���� |
1g |
0.25g |
0.12g |
0.25g |
0.25g |
������ |
1000mL |
(1)���������ڣ�ֲ�����ή����������ܵ�ԭ����__________��ʹֲ��ָ���������Ĵ�ʩ��_________��
(2)����ȥ����Һ�е�����þ��ֲ��ҶƬ����ɫ�仯��________________��
(3)Ҫ�뱣֤ˮ��ֲ��������������Һ������Ӧ����_________��Ԫ��(C��H��O����)��
(4)���������о���������Һͨ���������Ŀ����__________�������ڿ���Ԫ�ص����ա�
(5)Ҫʹ����ֲ���������ã����˹�������Ŀ���Ӫ�������˵��¶��⣬����Ҫ_______��______��������
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010-2011ѧ�������С���У���ˡ��߶���һ�������������ﲿ�֣��������� ���ͣ��ۺ���
��22�֣��±�Ϊ��������ʱ���õ�һ�ַ���ȫ����Һ����Ҫ�䷽���Ը��ݱ������ݣ��ش��������⡣ÿ1000ml����ˮ�м��룺
| �ɷ� | KH2PO4 | KNO3 | Ca(NO3)2 | MgSO4 |
| ���� | 0��2g | 1��0g | 1��0g | 0��4g |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2012�������С���У���ˡ��߶���һ�������������ﲿ�֣������棩 ���ͣ��ۺ���
��22�֣��±�Ϊ��������ʱ���õ�һ�ַ���ȫ����Һ����Ҫ�䷽���Ը��ݱ������ݣ��ش��������⡣ÿ1000ml����ˮ�м��룺
|
�ɷ� |
KH2PO4 |
KNO3 |
Ca(NO3)2 |
MgSO4 |
|
���� |
0��2g |
1��0g |
1��0g |
0��4g |
��1��������Һ�������� Ԫ�ز��ǿ���Ԫ�أ� �����ܱ�ֲ���ٶ����õĴ���Ԫ�ء�
��2��������Һ�е�Ԫ������ ��ʽ��ֲ��ϸ�����յģ��������շ�ʽ��Ҫ��
���̡�
��3��������Һ��Ũ��һ�㲻�����ֲ������������Ƥϸ���� Ũ�ȡ��������Һ��Ũ�ȹ��ߣ�ֲ�������������������������ֽ����ԭ��
��
��4������Ƽ�С���ͬѧ�����������༼���������ѣ����ǰ��շ��ѶԿ������ϵ����������˱���Ӫ��Һ��Ȼ�������������о��������������������ּ�ʱ����Ӫ��Һ����һ�ܷ����糤���������ڶ���ȴ������ȱ��֢��ͬѧ�Ƿ�����ԭ��ȡ����Ӧ�IJ��ȴ�ʩ���յ��˺ܺõ�Ч�������ǵ����ܣ�������ȴ������ή������

�� �ڶ��ܣ����������ȱ��֢����Ҫԭ��
��ͬѧ�ǵIJ��ȴ�ʩ�� ��
�� �����ܣ����������ή�������ԭ�� ��
��IJ��ȴ�ʩ�� ��
��5�������ȱ��ij�ֿ���Ԫ�ص�Ӫ��Һ��������һ��ʱ���������ľ����������Ҷ���ȳ��ֻ����ߵ㣬��ȱ����Ԫ�ؿ����� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com