
(12��)�����Ա������ʽΪZW�ͣ���ë��ɫ��«�����ơ���«�����ơ���ɫ���֡���ë��«�������ɰ��Ի���B���ƣ���ë����ɫ��Ҫ����A�Ĵ��ڣ�ZBW��ZbW��Ϊ�����ӣ���ë��Ƿ�ë��һ�Ե�λ������ƣ������ɻ���d���������з�«����ë�������Ƿ�ë���ӽ�������£����������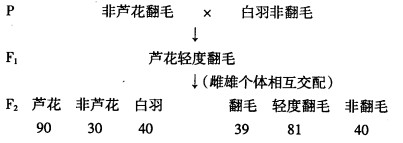
(1)��ë��ɫ��һ��״�Ŵ�ʱ�� �Ե�λ������ơ�������ë��ɫ��һ��״���ױ��Ƽ��Ļ������� ��F1���ۼ��Ļ������� ��
(2)F2Ⱥ����B�Ļ���Ƶ���� ��«�����Ļ������� �֣����д���֮��Ϊ ���������Ӻ�����ռ�ı����� ��
(3)��ë���Ƿ�ë�����״�Ŵ�ʱ���� ���ɡ����ж�B��b��D��d�Ŵ�ʱ�Ƿ���ѭ�����������϶��ɣ��۲�ͳ��F2�з�ë��(��Ƿ�ë��)�е��Ա�������ɵ�֪���� ����B��b��D��d�Ŵ�ʱ����ѭ�����������϶��ɣ�
�� ����B��b��D��d�Ŵ�ʱ��ѭ�����������϶��ɡ�
��1���� AAZbW AaZBZb
��2��2/3 6 1��2 1/4
��3��������� �����൱��˵��D��dλ�ڳ�Ⱦɫ���ϣ�
�Ա��Ϊ1��1��������ϣ����Ա�ȫΪ��һ��״��b��d������
��ë������Ƿ�ë����Ϊͬһ�Ա���ȷ�ë���д��������Ա����ӽ�1��1��˵��D��dλ����Ⱦɫ���ͬԴ�����ϣ�
���������������1�����ݡ���ë��«�������ɰ��Ի���B���ƣ���ë����ɫ��Ҫ����A�Ĵ��ڡ����жϳ���ë��ɫ��һ��״�Ŵ�ʱ�����Ե�λ������ƣ���ΪF1�ж���«����ë�������ױ��Ƽ��Ļ�������AAZbW���ۼ��Ļ�������aaZBZB����F1���ۼ��Ļ�������AaZBZb���Ƽ��Ļ�������AaZBW��
��2������F1���ۼ��Ļ�������AaZBZb���Ƽ��Ļ�������AaZBW����F2Ⱥ���и���Ļ�������12�֣������ǻ���Bʱ��4�֣��ֱ�ΪZBZB��ZBZb��ZBW��ZbW�����F2Ⱥ����B�Ļ���Ƶ����4/6=2/3��F2Ⱥ����«�����Ļ�������6�֣��ֱ�ΪAAZBZB��AAZBZb��AAZBW��AaZBZB��AaZBZb��AaZBW�����д���֮��Ϊ 2��4=1��2������Ļ������У�aaZBZB��aaZBZb��aaZBW��aaZbW������ZBW��ZbW��Ϊ�����ӣ����������Ӻ�����ռ�ı�����1/4��
��3�����ݷ�ë��Ƿ�ë�ĺ��F1���Ƿ�ë����F2Ⱥ���з�ë����ȷ�ë���Ƿ�ë=1��2��1������������ͱ�ΪDD��Dd��dd=1��2��1�����ϻ���ķ��붨�ɣ���Ϊ��ë��ɫ�ɰ��Ի���B���ƣ������ë��Ƿ�ë����Ҳλ����Ⱦɫ���ϣ������Ǻ�����۲��൱������B��b��D��d�Ŵ�ʱ����ѭ�����������϶��ɣ������ë��Ƿ�ë����λ����Ⱦɫ���ϣ������Ǻ������Ӧ�൱������B��b��D��d�Ŵ�ʱ��ѭ�����������϶��ɣ�
���㣺���⿼������֪ʶ�����ڿ���ѧ��������ѧ֪ʶ��Ҫ�㡢��ȡ��Ϣ�Լ�ʶͼ����������������ѧ֪ʶ�������⡢��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ͼ�ױ�ʾ���仡��3����Ԫ������ϵ�����С���<��ʾ����ͻ��ϸ�����ٵ���ͻ��ĩ�� (��һ����������Ԫģʽ)��ͼ�ұ�ʾͻ���������ṹģʽͼ�����ͼ�ش�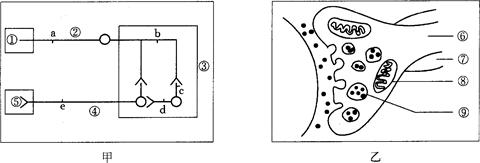
��ͼ���У����ٴ���С���ϵĸ����������(������ĩ�Ҽ���֧������ϼ���)��Ϊ_______________���۳�Ϊ_______________��������____������ͼ�ҵĽṹ��
��ͼ���У��̼�d��ʱ�ô�ϸ��Ĥ����ĵ�λ����Ϊ___________________��
����ͼ���У��˷��ڢڴ�����������������ʽ����.
���˷���ͼ�ҽṹ�е����ݵ�ԭ���� ��
��5����ͼ���б���˷ܴ��ݷ���
��6�����������У�ʹ��ij�־ֲ�����������ʹͼ�ҽṹ��_______________���ͷŵ�_______________�����仯���Ӷ���ʱʧȥ�˷ܴ������ܡ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ͼ��ʾϸ�������л������ɣ�������Ҫ���ܣ������ش�
��1�����A��B��C��D�Ĺ�ͬԪ���� ��
��2������C���ɸ����ںϳɺ�����������G�����У�������200��ˮ���ӣ���C����ĿΪ ����
��3��С������ϸ��������E��ָ ��ϸ�����ڵ�Ⱦɫ����Ҫ����ͼ�е� ���ɣ�����ĸ���ţ���
��4����ͬ������E��F���������ֽ⣬���������϶���� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��7�֣���ͼ�мס��ҡ�����������̬ϵͳ�ijɷ֣�A~F������ͬ�����ʣ���ͷ��ʾ̼ѭ����;�����������̡�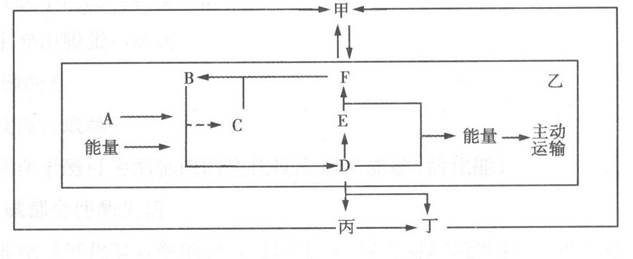
(1)���붡ϸ���ڣ������Է���ͼ�� �������̣�����ĸ�ͼ�ͷ��ʾ����
(2)B��_______________��D��___________ ������_______________ �����о�B��D���ʱ仯�ľ�����̡�
(3)����������Dȫ��Ϊ�����ǣ�������Ϊa mol��������Ҫ��F������ ___________ ������ʱ�Ҳ���b mol��F���� �����ǵĻ��������Ϊ ___________ ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��12�֣��ش������й�����������Ϣ���ݺ͵��ڵ����⡣��ͼ��ʾ������ϵͳ������Ŀ��ƣ���������ڡ�����Լ������һ�ֻ��μ��⣬���ֲ��ڰ��ױڡ����ͼ�ش�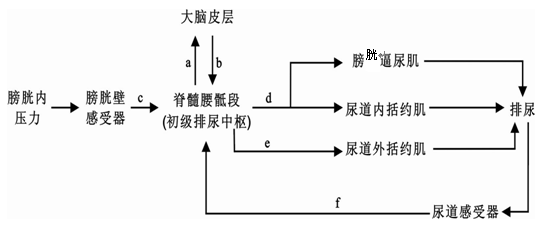
��1��ͼ������ڡ�����Լ�������ױ������ڷ��仡�е���һ���֣�_______________��
��2�������׳�ӯ���˻�������⣬���ü�ͷ��ͼ�б�Ҫ���֡���ĸ��ʾ�������������;���� ���������嶯�����Ĺ����У�c����άĤ���γ��� ������ҡ�������ľֲ�������
��3������Һ�������������������˷ܣ��˷ܴ�������������࣬��һ����ǿ��������Ļ������һ�� ������������������������ڡ�
��4����������£��˵�����������ʱ����������-��Һ���ڣ���ˮ���������ʧˮ����ʱ��ϸ����Һ��ѹ�� ���̼� ���ṹ���ƣ���ѹ��������ʹ �����ʸУ�ͨ��������ˮ����ˮ�֡��������ڸ���ԭ��������ʱ������쳣�����磺�ܵ����ź���ʧ����ԭ���� ��ij���ڳ������������ܵ���ʱ�����ˣ����ֶ���֢״��ԭ���ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
�����\��ʵ����10�֣������ǽ��ı���������ҶƬͨ��ֲ����֯�����γ�ֲ���ʾ��ͼ�����ͼ��ش���� ���⣺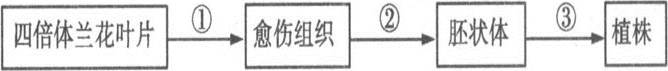 (1)ѡȡ�ı�������ҶƬʱ��Ҫѡ�� __________ ��ҶƬ����ֲ��������ſ��� __________ ���Ҵ˲��������ھƾ����Խ��С�
(1)ѡȡ�ı�������ҶƬʱ��Ҫѡ�� __________ ��ҶƬ����ֲ��������ſ��� __________ ���Ҵ˲��������ھƾ����Խ��С�
(2)ֲ�D���� __________��___________������ϸ�����ѵĹؼ��Լ��أ���ٽ���ȣ��ڽ� __________ ��������ƣ������ó��ּ�ǿ�����ơ�
(3)�ִ�ҩ���о������������ķ���ɷ�--������ʹ���Ŀ�����������������������ƣ��������ԡ�ʵ������ȡֲ�﷼���͵ķ���������____��__________ �ȣ��������������ԭ�Ϸ��õ�λ�ã����Խ�ˮ����������Ϊ __________��__________ ��__________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
ֲ��ĸ��������������������ԣ��������о�����������������������ʵ�顣
ʵ��һ�����հ���֬�ͺ�EDTA����֬����ñ״���ֱ����ڼס������������߸��ĸ����⣨ʾ��ͼ���£�����ʾ��EDTA��������ȥ�������ٽӲ�λ��Ca2+��
��1�����߸�ˮƽ��������һ��ʱ��۲쵽�����߸�������(��)�����������߸�ˮƽ������
��������ʵ�������ý��ۣ� ��
��2��ʵ�����ˮƽ���õ��߸�������������������ڽ��ز��Ca2+Ũ�����Ը���Զ�ز��йء��о������������dz���Ca2+Ũ�ȸߵķ�������������Ϊ��֤��һ���ۣ��������������ʵ�鷽����������ṩ��ʵ����Ϻ��þߣ�д���ڶ������Ժ��ʵ�鲽���ʵ���������ش����⡣
ʵ����Ϻ��þߣ��߸��ȳ����ȷ��������ӣ���EDTA����֬ñ����Ca2+����֬�飬�հ���֬�飬������ȡ�
ʵ�鲽�裺
��һ����ȡ���ɸ���������ÿ���������з����������ȷ��������ӣ���ʵ��һ������ķ��������߸�һ��ʱ�����ȥ���������֬ñ��
�ڶ�����
��������
���IJ���
����ͼ����˵����
��3��ʵ������ ��
(4)��ѧ�ҽ�һ��֤ʵ�������������صķֲ���Ca2+Ũ��Ӱ�졣���߸�ˮƽ����ʱ�������յ�Ca2+������²��ƶ������½��ز��������Ũ�ȱ�Զ�ز�ߡ�������߸��������طֲ��������������Ĺ�ϵ�� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��7�֣������о�֤ʵATP���ǡ�����ͨ������Ҳ����Ϊ��ϸ������Ϣ�����е�һ���źŷ��ӣ�����Ϊ�źŷ��ӵ����û�����ͼ��ʾ��������ش��й�ø�����⣺
(1)�������������������У���ֱ��������ATPˮ�����( )
A.θ����ø�ĺϳ� B.ϸ�������� C.������������ D.��ϸ������������
(2)��ͼ��֪��ϸ����϶�е�ATP���й�ø�������£������������������������ʣ�µ���_____________��
(3)һЩ��ϸ���������ͷŵ������ʣ������ͷ�ATP�����߾�����������ϸ����Ĥ��λ�仯����ͼ��������ѧ�ҵ����Ʋ�ATP����Ϊ��ϸ���䴫����Ϣ���źŷ��ӵ�ʵ��˼·�ǣ� _________________________________________ _ ��(����д��һ��˼·)
��4��ø�ǻ�ϸ�������ľ��д����õ��л������ɵ�λ�� ��
��5��ø֮�����ܹ����ٻ�ѧ��Ӧ���ٶ�����Ϊ���� ��
��6����ͼ���Ա�ʾ���� ��37��������ø�ٷ�Ӧ�ٶ���pH�Ĺ�ϵ����ѡ��
A.��Һ����ø
B.θ����ø
C.��֬��ø
D.��������ø
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ͼ��ijֲ�������ú�ϸ����������ʾ��ͼ��I��VII�������ʣ��ٵ��۴������̡���ͼ�ش��������⡣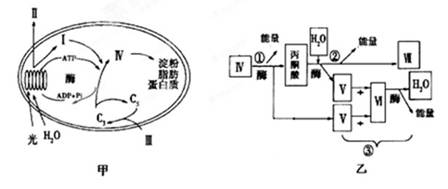
(1)�ڼ�ͼ�У�����CO2�ij�����____________����ǿ����ATP���ƶ�������____________________________________________________��
(2)�ڹ��������£�Ҷ��ϸ��������ADP�ij����� ____________________________��
������ֲ����1����CO2Ũ��ͻȻ����0��3��CO2Ũ����(����ǿ�Ȳ���)����ͼ�е�C3�ĺ�����_______________��C5�ĺ�����________________��
(3)��ͼ���������龥ϸ����ȱ��ʱ�Ĵ�л��ȣ����еIJ�����_________(������)����Щ
���̷�����___________________________��λ��
(4)����18O��Ǽ�ͼ�е�H2O������ϸ�����������������Ȳ�����Ե���__________��
�ס�����ͼ�в�ͬ���ִ���ͬһ���ʵı����__________________________��
(5)�����˵��¶Ⱥ���ǿ���£�����ֲ������ܱյIJ��������У��ⶨ��ʼʱCO2����
5.0g��12h��CO2����1��9g����ô12h�ڿɻ���������Լ__________g��(�������һλС��)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com