
��̥��ֲ�Ļ�����������ͼ(��ţ��̥��ֲΪ��)�����ͼ�ش����⣺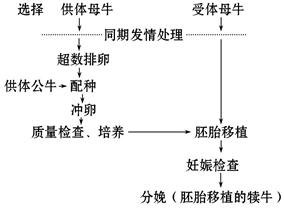
(1)�Թ���ĸţ�����幫ţ��Ҫ���� ��
(2)ͬ�ڷ��鴦���ķ����� �������еġ��ѡ���ָ ��
(3)�ܾ��йع����У��ٵ�һ�����ѿ�ʼ�����ͷŵڶ����壬�۶��巴Ӧ���ܴ�Խ�������ݴơ���ԭ�˵��γɣ���Ĥ��ʧ���ơ���ԭ���ںϡ�����ȷ��˳��Ϊ (�����)���ܾ��ı�־�� ��
(4)������ĸţ��������������״����������ϸ����¡���������Ŵ��������̣���ش������й����⣺
��Ŀǰ����ֲ�������ձ�ʹ�õ�ȥ�˷����� ��
�����ں���ֲ����ϸ��һ��ѡ�� �����ڵ�ϸ����ԭ���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��һ������������ʵ��С������Ⱥ��,żȻ���ּ�ֻС���ڳ����ڶ��ܺ�ʼ��ë,�Ժ�����������ë״̬��Ϊ�˽����״���Ŵ���ʽ,�о���������6��С�������,ͳ����ͬʱ����ڷ�ֳ������¡�
| ��ϱ�� | �� | �� | �� | �� | �� | �� | |
| ������� | ��� | 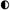 ���� ���� | 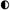 �� �� | ����� | ����� | ����� | |
| ����� | 6 | 6 | 17 | 4 | 6 | 6 | |
| �Ӵ�С�� ����(ֻ) | ��ë | 9 | 20 | 29 | 11 | 0 | 0 |
| ��ë | 12 | 27 | 110 | 0 | 13 | 40 | |
 �Ӻϡ�,
�Ӻϡ�, �Ӻ� ��
�Ӻ� ���鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
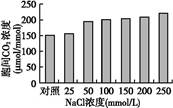
Ϊ̽���ζ�ij����ȼ���������������õ�Ӱ��,�ڲ�ͬŨ��NaCl ������,���侻������ʡ�����CO2Ũ�ȡ����ɫ�غ����Ƚ��вⶨ,�����ͼ������ڼ�ϸ���ĺ���ǿ��û�������仯�������ͼ�ش���������: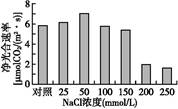
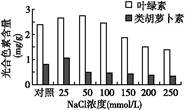
(1) Ҷ������ɫ�صĹ����� ��
(2)�����е�CO2��ͨ��ֲ��ҶƬ����� ����ֲ�����ڡ�������ò������л���(C6H12O6)�е�����Դ��ԭ���е� ,�л���(C6H12O6)�е�����ϸ�������������ղ��� �С�
(3)��NaClŨ����200��250 mmol/Lʱ��������������½�,��Ȼ�����¸�ֲ�����ļ����ʵ����羻�������Ҳ������½�������ǰ����Ҫ������ ,������Ҫ������ ��
(4)�ܹ�����ʿ��õ�λʱ���ڵ�λҶ����� ��ʾ��������������ͼ�ϻ��Ƹ�ʵ�����ܹ�����ʱ仯���Ƶ�����ͼ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ͼ�мס�����ͼ����װ��ʾ��ͼ,��ͼ�Ǹ�ëϸ��ʾ��ͼ������ݼס��ҡ�����ͼ�ش���������:(ͼ���Ƿ��������õij�ʼ״̬,ͼ����ͼ�����˽ϳ�ʱ���������֮��ﵽ��ƽ��״̬)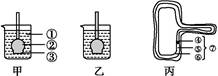
(1)���͵���װ�ñ���߱���������:
�� ;
�� ��
(2)�Ƚ�ͼ���Тٺ͢ڴ���ҺŨ�ȵĴ�С:�� (����ڡ�����С�ڡ��������ڡ�)�ڡ�
(3)ͼ���Т�Ϊ ,������ϵͳ�е������൱��ͼ���е� ��
(4)ͼ��ͼ���ж��а�Ĥ,��������䱾�ʵ�������ϸ��Ĥ��Ϊ����Ĥ���� ��,ϸ��Ĥ����һ��������Ĥ�� �йء�
(5)���Ѹ�ëϸ��������������Ϊ30%��������Һ��,�������ʲô�仯? �����Ѹ�ëϸ��������������Ϊ5%��NaCl��Һ��,�������������۲�һ��ʱ����ܻᷢ��ʲô�仯? ��
(6)�μ���е�ֲ�ﳣ����ή������,��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
Ϊ̽��NaCl��CuSO4����Һ����ø���Ե�Ӱ��,ijͬѧ������ʵ��,ʵ�鲽��ͽ����������ش�:
| �Թܱ�� ʵ�鲽�� | 1 | 2 | 3 | 4 |
| 1% NaCl��Һ(mL) | 1 | | | |
| 1% CuSO4��Һ (mL) | | 1 | | |
| 1% Na2SO4��Һ(mL) | | | 1 | |
| ����ˮ(mL) | | | | 1 |
| pH6.8����Һ(mL) | 1 | 1 | 1 | 1 |
| 1%������Һ(mL) | 1 | 1 | 1 | 1 |
| ��Һ����ø��Һ(mL) | 1 | 1 | 1 | 1 |
| ���Թܷ���37 �����ˮԡ��������ʱ�� | ||||
| ȡ���Թ�,����1%����Һ0.1 mL | ||||
| �۲��� | ��ɫ | ����ɫ | dz��ɫ | |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ͼΪ���ֺ�˹̹С��ţ�ķ�ֳ���̣����ͼ�ش��������⡣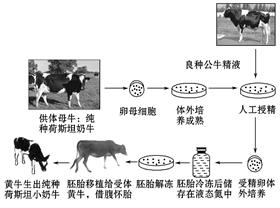
(1)��¡��������������ͼʾ��ֳ������ȣ����еļ����ֶ���______________ ������
(2)�ܾ��������������������Һ�ɷ�һ��Ƚϸ��ӣ���һЩ���κ��л������⣬����Ҫ����ά���ء����ء������ᡢ�������Լ� �����ʡ�
(3)�������ת�����˹̹��ţ���ɲ��� ������ҩ�õ������� �У��ڼ������ϸ���е�Ⱦɫ��DNA���Ƿ������Ŀ�Ļ���ʱ�������õķ����� �����������Ƿ���ʾ���ӽ������м�����
(4)���ֺ�˹̹��ţ����̥���������ţ���ӹ��ڴ���������������ԭ���� _________________________________________________________________
_________________________________________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
����ǿ������(MEOR)������ijЩ�����ܽ���ʯ�ͣ�����ʯ�͵��黯�ȣ�����ʯ���ȵ�ԭ����ͨ�����;���ע�뺬�����ˮ����߲����ʵ��¼���������������ɸѡ�������������������������ʵ���������ش��������⣺
(1)����������Ĵ�Ӫ����������Ӧȡ��____________________________ ��������
(2)Ϊɸѡ�ʹ�����������ӹ����Ϸ֣�Ӧѡ�� �����������õĽ��ַ�����__________________________________________________________
�� ��
(3)���ֺ�Ӧ�ܷ�������ԭ���� ������һ��ʱ��������������γɽ���Ȧ����ʱѡȡ_____________________________
�Ϳɻ�ø�Ч���ꡣ
(4)����������������Ҫ��������м�����ȡ0.2 mL��Ʒ���ù����������������������ͳ������ƽ�壬�������ֱ���200��180��220������Ʒ�����������Ϊ ��/mL����ͳ�ƽ�� (����ڡ����ڡ�)ʵ��ֵ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��Ӭ���з�ֳ�ٶȿ졢�������ڶ̡�Ⱦɫ����Ŀ�١������ֵ���״����ص㣬���Ŵ�ѧ�о��ĺò��ϡ���ش������й����⣺
(1)Ħ��������X�������䴦�����۹�Ӭ��֮�����ܻ�ð��۹�Ӭ����Ϊ������ ��
(2)��ͼΪ��Ӭ��ԭʼ��ֳϸ����Ⱦɫ����ɼ�Ⱦɫ���ϲ��ֻ���ֲ���ʾ��ͼ���ù�Ӭ�Ļ������� ���ù�Ӭ����ܲ��� ����ϸ����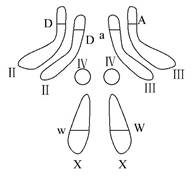
(3)һ��������ͼ��ʾ����ԭϸ��������һ��ADXW�ļ��壬��ô��ü���ͬʱ��������ϸ��������Ϊ ��
(4)��ͼ�Ǹù�Ӭ����Ⱦɫ��ṹ�����쳣ʱ���ֵ���������쳣���� ��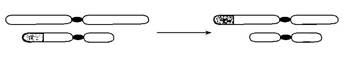
(5)��һ���ȶ��Ŵ��Ļ�����Ӭ��Ⱥ�У�������һֻ�����۹�Ӭ��������֪������������״�������ʵ�飬�жϺ���������λ�ڳ�Ⱦɫ���ϻ���λ��XȾɫ���ϣ��������йص�ʵ�鲽�衢���ܵ�ʵ��������Ӧ��ʵ����ۡ�
ʵ�鲽�裺
��һ�����ô��ֵ� �����ĺ����۹�Ӭ���䣬����һ������һ����������__________��
�ڶ���������һ���� ��ԭ��Ⱥ�Ļ����۹�Ӭ���䣬���Ӷ�����������ͳ���Ӷ�����ͬ�����͵ĸ�������
����ͽ��ۣ�
������Ӷ������嶼�ǻ�����Ӭ�����������λ�� Ⱦɫ���ϣ�
������Ӷ������ֺ����۹�Ӭ�����������λ�� Ⱦɫ���ϡ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
ͼ�ױ�ʾ�������ֲ�������ſ��ȣ�����һ��������������ϸ����ɵģ��ĵ��ڻ��ƣ�ͼ�ұ�ʾ��ij��ɫֲ�����һ�ܱ��������ڱ���һ���¶ȵ�����£����費ͬ��������ʱ��õ��ܱ�����������Ũ�ȳ����仯���ߡ����ͼ�ش�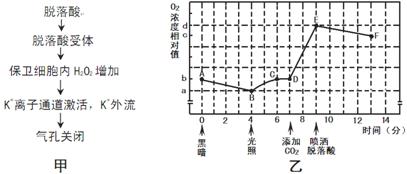
��1�����������йص�ø�ֲ��� ��
��2��A�������²���ATP�ij����� ��
��3������CD����ʾ�����ԭ���� ��
��4��7����ʱ��ֲ������CO2����ʱ����Ҷ������NADP+������ ��
��5������������Ļ�ѧ������ ��
��6������EF����ʾ�����ԭ���� ��
��7����ֲ��ҶƬ���������ᣬ��ʱ����Ҷ������RUBP�ĺ����� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com