
��ͼΪ����ij��֯��ʾ��ͼ�������ͼ�ش���������⡣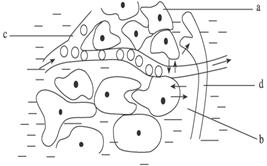
��1��ָ��ͼ����ָ��λ���ƣ�a��ʾ ��b��ʾΪ��֯��϶�� ��c��ʾΪëϸѪ���ڵ� ��d��ʾ���ܰ��ڵ� ��
��2������b��c��d�ϳ� ��������������ϸ�������Һ�廷�����ʳ� ��
��3�������ڵ�ϸ��ͨ�� ��������绷����ӵؽ������ʽ��������ڷ���ϵͳ����ϵͳ���³´�л���� ���á�
��4���˵�����ĥ��ġ�ˮ�ݡ��еĵ���ɫҺ����Ҫ�� ��
 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
��ʮ�������й���������ϣ��ش����⡣
1982��Ĵ�����ѧ�ߴ�θ�����֯�з���������ݸ˾���
61. �����ݸ˾����Ŵ����ʼ��зֲ��������Ϊ ��

62. ��ͼ4֧�Թֱܷ����4�������ڰ��������������֬����3.5g/L���е�����״̬�����Тں��Թܴ��������ݸ˾�������״̬����ͼ�жϣ��þ��� �����²���������������ϸ��������״̬�������Թ� ������
63. �±���ij���������䷽��
| �ɷ� | ������ | KH2PO4 | MgSO4 | NaCl | CaSO4 | CaCO3 | ��֬ | ����ˮ |
| ���� | 10g | 0.2g | 0.2g | 0.2g | 0.2g | 5g | 3.5g | 1L |
�������ݸ˾����ֵ�pH���˵ĸ��������У�����37��������һ��ʱ����ڸ��������������ݸ˾�����Ŀ�ȸս���ʱ ����Ҫԭ���ǣ� ��
�����ݸ˾���̬����ͼ��ʾ���þ��������п�����θ�����θ��������

64. �����ݸ˾�����������pHΪ6��7������θǻ��pH��1��2֮�䣬��θճĤ��ճҺ�㿿����Ƥϸ����pHΪ7.4���ҡ��������ݸ˾���ʳ�����θǻ�������ṹ�ص��Լ��ܵ���θ��������ԣ��Ʋ�þ���θ����δ�
65. ���ݵ�ʮ������Ϣ���������ݸ˾��Ƿ����ڹ�ϸ���� ��
ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010��߿���������������֮ר��ʮ�� ���\��ʵ�� ���ͣ��ۺ���
��ʮ�������й���������ϣ��ش����⡣��10�֣�
1982��Ĵ�����ѧ�ߴ�θ�����֯�з���������ݸ˾���
61. �����ݸ˾����Ŵ����ʼ��зֲ��������Ϊ ��
62. ��ͼ4֧�Թֱܷ����4�������ڰ��������������֬����3.5g/L���е�����״̬�����Тں��Թܴ��������ݸ˾�������״̬����ͼ�жϣ��þ��� �����²���������������ϸ��������״̬�������Թ� ������
63. �±���ij���������䷽��
| �ɷ� | ������ | KH2PO4 | MgSO4 | NaCl | CaSO4 | CaCO3 | ��֬ | ����ˮ |
| ���� | 10g | 0.2g | 0.2g | 0.2g | 0.2g | 5g | 3.5g | 1L |

�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010���Ϻ��������������ڶ���ģ�⿼�������Ծ� ���ͣ��ۺ���
��12�֣��ڻ���������̬�������������������ı�Ҫ������
��1����������ά������̬����ؼ����ã���ͼʾ�⡰������---����ϵͳ����������ش�
1����֯������Ԫ֮����Ӵ��IJ��ֺϳ�Ϊ___________���˷�ͨ���ýṹʱ����Ϣ�Ĵ�����ʽ���仯������_________________��
2��ij�����ð��������ˮ����������ʹ��
_________________�½��������˶��������е�__________�������Ĵ̼���ADH�ķ��ں��ͷ���_________________�����´�������
������ά��ˮƽ�⣻ADH�ķ��ں��ͷ��������ܵ�ϸ����Һ��_______________�����ص�Ӱ�졣
3��ͼ���ұ�ʾ�����Ե������Ԫ���ܺϳɺͷ��� �����ش��弰�����й��ڷ����ٵĻ���������ܵ�����̼�ʱ��ͼ����ʾ�ļ���A��B�ķ������������ӣ����м���B���������ӵĵ��ڷ�ʽ�� ������B���õİ�ϸ��Ϊ________________��
��2��ѪҺ��ά�ֻ�����ڻ����ȶ��Է���������Ҫ�Ĵ�����Ϣ���á��±���ij�������ӵ�ѪҺ���鵥�в������ݣ���ݴ˷�����
ѪҺ���鱨�浥
| ������Ŀ | ������ | �����ο�ֵ/mmol��L-1 |
| �ܵ��̴� | 7.80 | 3.60��6.50 |
| �������� | 14.65 | 0.45��1.18 |
| ���ܶ�֬���ף�HLD�� | 0.85 | 0.90��1.68 |
| ���ܶ�֬���ף�LDL�� | 4.02 | 2.84��4.10 |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2014�찲����У�����о����¸������ʲ���������������棩 ���ͣ��ۺ���
Ѫ��������������������Ҫ��Դ�����������������Ѫ�Ǻ������ֶ�̬ƽ�⡣������ش�����Ѫ��ƽ����ڹ��̵��й����⣺


��1��������ͼ��ʾijͬѧ��ʳ��Ѫ�ǡ��ȵ��ء��ȸ�Ѫ���غ����ı仯�����б�ʾ�ȵ��غ����仯���� ���ߡ�
��2����ͬѧ��ʳ��Ѫ������������ ����ʳһ��ʱ���Ѫ���ָֻ�������ˮƽ���������� �ķ������ӣ�����֯ϸ������� ��ϣ���ʹ��֯ϸ��
��
��3������ͬѧ��ѧ���ڲٳ����˶���һ��ʱ�䣬�˶�ǰ��Ѫ��Ũ�ȵı仯��������ͼ��ʾ��bc��Ѫ��Ũ���½���ֱ��ԭ���� ��cd��Ѫ��Ũ������������ �ķ������ӣ��ٽ��� �ֽ���������ǡ�
��4��������һ�������ȵ��ط���ȱ�ݻ��ȵ��������ϰ����µ��Ը�Ѫ��Ϊ�����Ĵ�л�Լ����������ж������ͣ�������һ�����͵������ߵ��ȵ��ط����������ͣ�������ƫ�ߣ�����֯ϸ���Զ��ȵ��ز������У�ԭ�������
��
��5����Ѫ��Ũ�ȸ���180mg/dLʱ����С���˹�Һ����ѹ���ӣ�����С�ܱ�ϸ���� ��С��Ӱ����С�� �Ӷ������������ࡣ
��6����С���˹�Һ�е�������Ũ��ƫ�ߣ���С�ܱ�ϸ���� ���������ޣ������˹�Һ�е������Dz���ȫ�����������գ���������������Һ�ų����⡣��Һ���Ƿ������ǿɲ��� �Լ���⡣
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010���Ϻ��������������ڶ���ģ�⿼�������Ծ� ���ͣ��ۺ���
��12�֣��ڻ���������̬�������������������ı�Ҫ������
��1����������ά������̬����ؼ����ã���ͼʾ�⡰������---����ϵͳ����������ش�

1����֯������Ԫ֮����Ӵ��IJ��ֺϳ�Ϊ___________���˷�ͨ���ýṹʱ����Ϣ�Ĵ�����ʽ���仯������_________________��
2��ij�����ð��������ˮ����������ʹ��
_________________�½��������˶��������е�__________�������Ĵ̼���ADH�ķ��ں��ͷ���_________________�����´�������
������ά��ˮƽ�⣻ADH�ķ��ں��ͷ��������ܵ�ϸ����Һ��_______________�����ص�Ӱ�졣
3��ͼ���ұ�ʾ�����Ե������Ԫ���ܺϳɺͷ��� �����ش��弰�����й��ڷ����ٵĻ���������ܵ�����̼�ʱ��ͼ����ʾ�ļ���A��B�ķ������������ӣ����м���B���������ӵĵ��ڷ�ʽ�� ������B���õİ�ϸ��Ϊ________________��
��2��ѪҺ��ά�ֻ�����ڻ����ȶ��Է���������Ҫ�Ĵ�����Ϣ���á��±���ij�������ӵ�ѪҺ���鵥�в������ݣ���ݴ˷�����
ѪҺ���鱨�浥
|
������Ŀ |
������ |
�����ο�ֵ/mmol��L-1 |
|
�ܵ��̴� |
7.80 |
3.60��6.50 |
|
��������[��Դ:Zxxk.Com] |
14.65 |
0.45��1.18 |
|
���ܶ�֬���ף�HLD�� |
0.85 |
0.90��1.68 |
|
���ܶ�֬���ף�LDL�� |
4.02 |
2.84��4.10 |
1�����ݱ��浥���ṩ����Ϣ�������ж������ӻ���֬Ѫ֢�������������ܵ��̴��߳������Ŀ���ԭ����__________________________________________________________��
2������ĵ��̴��������Ѫ�ܱ��ڲ࣬����Ѫѹ���ߡ�����Ѫѹ����Ҫ��λ��_______������ѹ��ֵ��������ѹ������________________��ɵġ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com