
��8�֣���ĸ�����������ڼ������յ������»����ˣ������������һ����Ĥ�����������䷢�������Եġ����ɡ������õ����ʵ���������Ϊ �������塱������ø�����γɡ�������ø�塱���ձ����⡣������
��1��Ϊ�۲�����ϸ���е������壬���� Ⱦɫ����Ⱦɫ��ϸ��һ�� �����ǡ����ǡ�����������״̬��
��2����������Ĥ�ṹ�Ļ���֧���� ����������ø�塱�γɵĹ���˵������Ĥ���� ��
��3����ij��ĸ������������⡰���ˡ��������������£������������ֽ���ղ����� ��������ʱ�������Լ��� ��
��4������˵ľ������������������ͻ�䣬���ֱ���һ�� �����ܡ����ܡ����Ŵ�������������� ��
��1�������̣�1�֣� �ǣ�1�֣� ��2����֬˫���Ӳ㣨1�֣� һ�������ԣ�1�֣�
��3���ƾ��Ͷ�����̼��1�֣� ��������̼:�����ʯ��ˮ;������ݷ���ˮ��Һ�����ƾ����ظ���ص�Ũ������Һ��1�֣� ��4�����ܣ�1�֣� �ܾ�ʱ������DNA���ܽ�����ϸ�������������ã���������λ�ھ��ӵ�β����һ�㲻������ϸ������1�֣�
���������������������û���Ⱦɫ��������Ⱦ������ɫ����ϸ�����ֻ��ԡ���2������Ĥ�Ļ����Ǽ�����֬˫���Ӳ㡣���������ø�嶼������Ĥ�ṹ���ܷ����ںϣ���������Ĥ���ںϣ�˵������Ĥ����һ�������ԡ���3�������������������������εķ�Ӧ������������ˣ����ĸ�����ܼ�������������ֻ����ϸ���ʻ��ʽ�����������������Ϊ�ƾ��Ͷ�����̼����������̼:�����ʯ��ˮ���ǣ�������ݷ���ˮ��Һ���������ٱ�ơ����ƾ����ظ���ص�Ũ������Һ�����ɫ����4��������λ�ھ��ӵ�β����һ�㲻������ϸ���������Ŵ��������
���㣺���⿼��ϸ���ṹ�������֪ʶ�����ڿ��鿼����������ѧ֪ʶ��Ҫ�㣬����֪ʶ���������ϵ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��6�֣����ش������й�ϸ����ϸ�����ڵ����⡣ͼ1Ϊϸ�����ڵ�ģʽͼ��ͼ2Ϊ����ϸ���������ṹģʽͼ����ͼ�ش𣨷�������ͼ�е����֣������������֣���
��1����ͼ1�У�X�ڴ���____________��ϸ����X�ں�ӱ���ϸ������____________�������Ⱦɫ����Ŀ���쵼�µ��Ŵ���ʱ����ѡ��ۡ���____________�ڵ�ϸ����1��ĩ��ʧ��ϸ���ṹ��____________��
��2�����鵰�������Ⱦɫ��ĵ����ʡ�ͼ2�У��鵰�������ʱ���ܷ��������Եij����ǣۡ���____________�ͣۡ���____________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��10�֣���ϸ�۲����и��ֽṹģʽͼ����ͼ�ش����⣺
��1��������ͼ�ṹ�й��е������� ��
��2��AͼΪ����0.3g/mL������Һ�г����ʱڷ����ϸ��������ϸ������ �����ţ������������¡�
(3)������ά��ø����A��B��C����ϸ������ͼ��ϸ����㷢�����Ա仯���� ��
(4)Aͼ��ʾ��ϸ���ʱڷ��븴ԭ�����������������ѷֻ�����ֳ�����л���������ʧ�Ľṹ�� ��д�ṹ���ƣ���
(5)Dͼ��ʾ���� ����/���ܣ����ж�����л��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��8�֣�ij��ɽ����нǺ�����һ�������״����һ�Ի������(A��������нǡ�a ���������)�����ö�Դ��ϵ��нǹ������ĸ���ӽ����õ��㹻�����һ�������й���ȫΪ�нǣ�ĸ��ȫΪ�ǡ�F1���۸�������䣬��F2�����У��нǣ���=3��1��F2ĸ���У��нǣ���=1��3��
��1���������ʵ�������������Ľ��ͣ�
��A��a����λ�� Ⱦɫ���ϣ�
���ڹ����У� �����;����нǣ� �����;����ǣ�ĸ���У� �����;����нǣ� �����;����ǣ�
��2�����������ͳ�����F2��ĸ���еĻ����ͼ������� ��
��3��Ϊ����֤��1���Ľ����Ƿ���������ǹ����F1�еĶ�ֻ��ĸ���䣬���Ӵ� ����1���Ľ��ͳ�����
��4������̽�������������ִ���ѧ�о��г��õ�һ�ֿ�ѧ���������� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��16�֣����ھ��÷�չ���˿���������ˮ�ŷ������ӡ�Ϊ���������������ˮ���⣬ijˮ��������ˮԴ��̬ʪ�ء�����Ϊ�˹�ʪ��Ⱥ����ɼ�ͼ�����ͼ�ش����⣺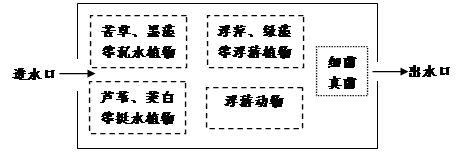
(1)������˹�ʪ�ص������� ��
��2��ʪ����«έ������ͺ����ֲ��ķֲ����ã�������Ⱥ��� �ṹ��ʪ���е�«έ����Ƽ�����ζ�������ﹲͬ���� ��
ij������Υ���ŷŴ�����ˮ�����ָ��ζ�����������һ����������Ⱦ���������¸��������������ù������� ���ڡ�
������ˮ�����˹�ʪ�ص����ʣ����������л���Ⱦ�ﱻ��ַֽ��⣬�������� ��ʹ��ˮ�ڴ���ˮ�ʴﵽ�ŷ�Ҫ��ˮ���ܹ��������˹�ʪ�أ�˵����̬ϵͳ�� ��
��5������ͼ�У��ü�ͷ�ͱ�Ҫ�����ֱ�ʾ��������������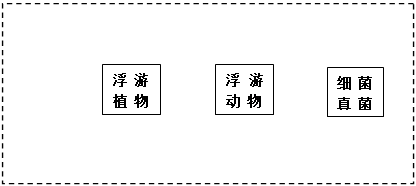
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��7�֣�ij�о�С����������������˹������Ӱ�����ص��о���ʵ�������±�����ش����⣺
| pH | ������������ ��2.0g/L�� | ������������ ��1.0g/L�� | ������������ ��0.5g/L�� |
| ����������mg/L��h�� | ����������mg/L��h�� | ����������mg/L��h�� | |
| 3 | -0.117 | -0.027 | -0.043 |
| 5 | 1.242 | 0.706 | 0.364 |
| 6 | 0.976 | 0.557 | 0.287 |
| 7 | 0.722 | 0.413 | 0.213 |
| 8 | 0.537 | 0.303 | 0.156 |
| 10 | 0.087 | 0.052 | 0.026 |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
�������ķ�������ʮ�ָ��ӣ���ͼ�Ǣ��͡��������������ַ�������ʾ��ͼ����ش�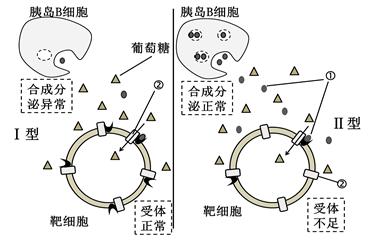
��1��ͼ�Тٱ�ʾ�������� ���ڱ�ʾ�Ľṹ�� ����������£���Ѫ��Ũ������ʱ���ṹ�ڻ��ǿ����ͼ������Ӱ��ڷ������õ���Ҫ���ʺͽṹ��ATP�� ��
��2�����ͼ�а�ϸ���Ǹ�ϸ������Ѫ��Ũ�Ƚ���ʱ�������ڸ���ϸ���ļ�����Ҫ�� ��
��3���о�����ij��ԭ�Ľṹ�����ȵ�Bϸ��Ĥ��ij�ṹ������ÿ�ԭ�̼����������������Կ��幥���ȵ�Bϸ�������µ������� ������ͻ���ͣ�����������ѧ�Ƕȷ����ü������� �������ٴ��� ������ͻ���ͣ�������ͨ��ע���ȵ������ơ�
��4������15N��ǵİ������о������ʺϳɺͷ��ڵĹ���ʱ���������γ��ַ����Ե�ϸ������ ��
��5������������ȵ�Bϸ���ֱ�����ں���5.6 mmol/L������(������)��16.7 mmol/L������(������)������Һ�У�����һ��ʱ�����м�⣬�������ͷ��ȵ��ضࡣ�˽��˵��______________________________________________________��
��6������ϸ����������������֤�ȵ�Aϸ���ķ������ܴٽ������ȵ�Bϸ�������ȵ��أ��������£�
���ø�������Һ�����ȵ�Aϸ����һ��ʱ�����˵õ�ϸ������Һ��
���ú�����Һ������Һ�����ȵ�Bϸ����һ��ʱ���ⶨ����Һ���ȵ��صĺ�����
��ָ�����������Ĵ���
��________________________________________��
��________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��24�֣���������ijЩ�л����Ԫ����ɿɱ�ʾ���£�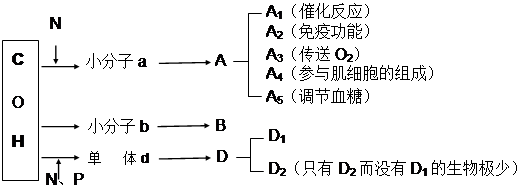
��1��ͼ��a�ĽṹͨʽΪ����������,
��2��A�ɷ�ΪA1��A2��A3��A4��A5����a����A�����Ե�ԭ����____________________________________________________________��
��3������A�Ĺ����жϣ�A3������Ϊ__________��A5������Ϊ________��
��4�����B������ĸ���ԭ����b��______��
��5�����B����Ҫ�Ĵ������ʣ���B��_______��������b(֬�������)�γɵġ����������⣬ϸ���е�֬�ʻ����� �� �� �� ��
��6��D���Ŵ���Ϣ��Я���ߡ�������ϸ���У�����d���� �֡�D1������Ϊ________
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��13�֣���ͼ��ʾij������Ľṹʽ����ͼ�ش�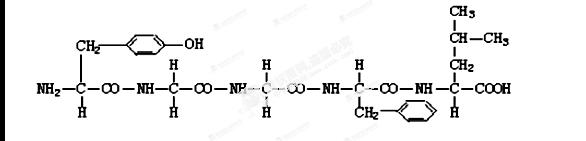
�Ÿû������� �������� ���Ȼ���
(2)�û��������� ��������ʧȥ ����ˮ���γɵģ�ˮ�����е��������� ���������� ��������Ӧ���γɵĽṹ��CO��NH������________ �� �˻����ﹲ�� �������Ľṹ�� �û������ �ġ�
(3)�û������� �ְ����ṹ�ɣ���ɰ��������ͬ��ԭ���� ��ͬ��
����д��������Ľṹͨʽ�� ��
(5)����һ����m�������ṹ�ɵĺ���n�������ĵ����ʷ��ӣ���֪�������ƽ��������Ϊa��д����������ʵ���Է��������ı���ʽ_______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com