
�ȵ���������������Ҫҩ���ͼ�����û��̼����������ȵ��صIJ�������ʾ��ͼ�����ͼ����?
��1���ܷ������˵�Ƥ��ϸ������ɢٹ��̣� ��Ϊʲô�� ��
��2��Ϊʹ���̢�����У����� ����D��ϸ���ڡ�Dϸ��һ��ѡ�ô˾���ݲݸ˾���ԭ���� ��
��3����ͼA��D�α�ʾ������ijƬ�Σ���B��ʾ�����ӣ�ΪʹĿ�Ļ���ֱ�ӱ��Ŀ�Ļ���C��������λ����ͼ�е� ������������ţ���?

��4���������������Ӧ�ã��类��������ת�ȵ���ø���Ƽ�������߲ˣ������Ը��߲˴������������ܸ���������ĸ���Ӱ����
��
 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
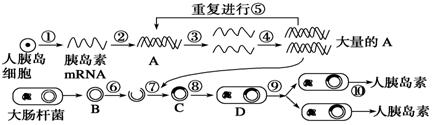
�ȵ���������������Ҫҩ���ͼ�ǻ��̼��������ȵ��صIJ�������ʾ��ͼ�����ͼ�ش�

(1)��ɹ��̢ڱ����ø��������ø��
(2)������ϸ���ȵ��ػ�����ȣ�ͨ���ڹ���ֱ�ӻ�õ�DNAƬ�β���������������������
(3)ȡ�Դ˾�������B���ڻ������������������á�
(4)���̢����õ�ø������������������
(5)Ϊʹ���̢�����У���������������������ϸ��D��ϸ���ڣ���������ͨ�ԡ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
�ȵ���������������Ҫҩ���ͼ�����û��̼����������ȵ��صIJ�������ʾ��ͼ�����ͼ����

��1���˵�Ƥ��ϸ�����Ƿ����ȵ��ػ��� ���ܷ������˵�Ƥ��ϸ������ɢٹ��̣� ��Ϊʲô�� ��
���̢ڱ����ø�� ��
��2����ij��Ѫ��Ũ��Ϊ10.6mmol/L����췢����Һ���������ǣ����˿��ܻ�ʲô���� ����λ���������ļ����������� ���ڲ�������ġ��ɹ���λ����ѡ���ʳƷ�У�����������������ƹϡ��۲ˡ��ɿ��������ϡ������λ���˵IJ��飬����Ϊ���ṩ��ʳ���飺
��λ����Ӧ�ö�Ե�ʳƷ�� �ȡ�������д�����֣�
��λ���˾����ٳԵ�ʳƷ�� �ȡ�������д�����֣�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010��2011ѧ�꽭��ʡ�Ͼ�ʦ���и߶���ѧ����ĩ���������Ծ� ���ͣ��ۺ���
�ȵ���������������Ҫҩ���ͼ�����û��̼����������ȵ��صIJ�������ʾ��ͼ�����ͼ����
��1���˵�Ƥ��ϸ�����Ƿ����ȵ��ػ��� ���ܷ������˵�Ƥ��ϸ������ɢٹ��̣� ��Ϊʲô�� ��
���̢ڱ����ø�� ��
��2����ij��Ѫ��Ũ��Ϊ10.6mmol/L����췢����Һ���������ǣ����˿��ܻ�ʲô���� ����λ���������ļ����������� ���ڲ�������ġ��ɹ���λ����ѡ���ʳƷ�У�����������������ƹϡ��۲ˡ��ɿ��������ϡ������λ���˵IJ��飬����Ϊ���ṩ��ʳ���飺
��λ����Ӧ�ö�Ե�ʳƷ�� �ȡ�������д�����֣�
��λ���˾����ٳԵ�ʳƷ�� �ȡ�������д�����֣�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010�꽭��ʡ�γ��и�����ѧ���������������� ���ͣ��ۺ���
��7�֣��ȵ���������������Ҫҩ���ͼ�����û��̼����������ȵ���ʱ����Ŀ�Ļ���Ĺ��̡����ͼ�ش�

��1�����Ǽ��ķ���֮һ���ڴ��������м���_______________�Լ���
��2�����������˵�Ƥ��ϸ������ɢٹ��̵�������_________��
��3�������ȣ��ڲ�ͬ�ļ����Է�ʽ��_________��
��4�����ȵ���mRNA�������ʡ�����ऺ������ʷֱ�ռ���������32%��26%��19%����B�к���������________%��
��5����C����1000������ԣ�����������400�����ظ����Т�ʱ����9000������İ�������������ᣬ��ݹ����ظ�������_______�Ρ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2012�������ʡ�߶���ѧ��4���¿������Ծ� ���ͣ��ۺ���
�ȵ���������������Ҫҩ���ͼ�����û��̼����������ȵ��صIJ�������ʾ��ͼ�����ͼ����?

��1���ܷ������˵�Ƥ��ϸ������ɢٹ��̣� ��Ϊʲô�� ��
��2��Ϊʹ���̢�����У����� ����D��ϸ���ڡ�Dϸ��һ��ѡ�ô˾���ݲݸ˾���ԭ���� ��
��3����ͼA��D�α�ʾ������ijƬ�Σ���B��ʾ�����ӣ�ΪʹĿ�Ļ���ֱ�ӱ��Ŀ�Ļ���C��������λ����ͼ�е� ������������ţ���?

��4���������������Ӧ�ã��类��������ת�ȵ���ø���Ƽ�������߲ˣ������Ը��߲˴������������ܸ���������ĸ���Ӱ����
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com