
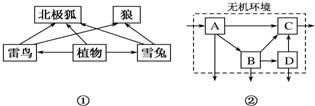
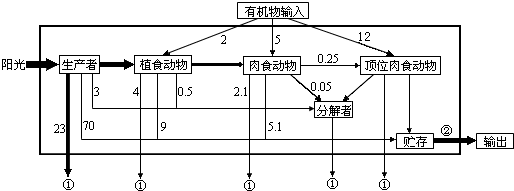
���� 1��ͼ���й���5��ʳ������������ռ�ж���������Ӫ������
2��ͼ��a�������ߣ�bd�������ߣ�c�Ƿֽ��ߣ�
3��ͼ���У��ٱ�ʾ�������ã��ڱ�ʾ�������л����е�������ͼ�������߹̶���̫���ܵ�ȥ�������ֲʳ����ͬ�����������ġ����ֽ������á�δ�������ĸ����֣���ֲʳ����ͬ������������ԴΪ������������ͬ��������粹�䣬ȥ�������С����ʳ����ͬ�����������ġ����ֽ������á�δ�������ĸ����֣�
��� �⣺��1��ͼ������5��ʳ������ѩ�ú���֮�������Ե��ּ��ϵ�Dz�ʳ��
��2���������⣺ֲ�����������80��$\frac{1}{8}$��10%=100kJ��ֲ����������������80��$\frac{1}{2}$��20%��10%=2000kJ��ֲ���ѩ�á���������80��$\frac{3}{8}$��20%��10%=1500kJ����ֲ�����ӵ�������3600kJ��
��3��ͼ���еĸ��ֳɷ�ͨ����������������ѭ������Ϣ���ݽ��ܵ���ϵ��һ���γ�һ��ͳһ�����壬c�õ��ļ�ͷ�࣬������̬ϵͳ�еijɷ��Ƿֽ��ߣ�
��4������̬ϵͳ�����Ƕ����ֲʳ����ͬ��������Ϊ��0.25+0.05+5.1+2.1-5.0=2.5��ֲʳ�����������ͬ��������Ϊ��2.5+4+9+0.5-2=14������̬ϵͳ�������߹̶���������Ϊ��23+70+3+14=110��103kJ/m2•y��=1.1��105kJ/m2•y����ʳ�����������Ϊ2.1+5.1+0.05+0.25=7.5������7.5����λ��������5�����������ģ�������ʳ�����ֲʳ�����õ�������7.5-5=2.5����ֲʳ�����������Ϊ2.5+4+9+0.5=16������ֲʳ��������������2����λ�����������ѱ�ͬ���������ȥ�����Եڶ�Ӫ����������Ӫ�����Ĵ���Ч��Ϊ2.5��16=15.6%��
�ʴ�Ϊ��
��1����ʳ
��2��3 600
��3���ֽ���
��4��110 15.625%
���� ���⿼������̬ϵͳ�Ĺ��ܵ����֪ʶ�㣬���ڿ���ѧ������ѧ֪ʶ�����������ճ̶ȣ�������ѧ������ͼ�Ρ���ȡ��Ϣ��������������������Ҫ��ϸߣ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | �����ڶ��η��Ѻ��� �����ڶ��η��Ѻ��� ������һ�η��Ѻ��� | |
| B�� | ������һ�η��Ѻ��� ������һ�η��Ѻ��� �����ڶ��η��Ѻ��� | |
| C�� | ���Ǽ�����һ�η��Ѻ��� | |
| D�� | ���Ǽ����ڶ��η��Ѻ��� |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | ȫ������ָ�Ѿ��ֻ���ϸ����Ȼ���з��������������DZ�� | |
| B�� | �����������е�ϸ���У��ܾ��ѵ�ȫ������� | |
| C�� | ��¡��ĵ���֤���˸߶ȷֻ��Ķ���ϸ������ȫ���� | |
| D�� | ��������ϸ��û�б��ֳ�ȫ���ԣ��ǻ������ض�ʱ����ѡ���Ա���Ľ�� |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2016��ɽ��ʡ������ѧ�ڵ�һ��ģ�⿼�������Ծ��������棩 ���ͣ�ѡ����
�����й����ʽ���ϸ���ķ�ʽ����������ȷ����
A��H+����ͨ��������ɢ��ʽ����ϸ��
B�������¶Ȳ�Ӱ��ֲ���ϵ�Կ���Ԫ�����ӵ�����
C���˼�״������ϸ��ͨ������������ȡ��
D�����������嵰��Э��������������ϸ����
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | ֦��Ǥ�� | B�� | ����ժ�� | C�� | ���� | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
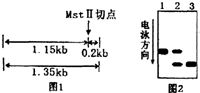 ��֪�������鵰������A��ʾ����Mst II����ø�и��ɵõ�����Ϊ1.15kb��0.2kb������Ƭ�Σ�����0.2kb��Ƭ��ͨ�����������쳣���鵰������S��ʾ������ͻ��ǡ����Mst II����ø�и���ϣ����ʧȥ�˸�ø��λ�㣬��Mst II����ø������ֻ���γ�һ��1.35kb��DNAƬ�Σ���ͼl������Mst II����ø�Ա��Ϊ1��2��3��������Ʒ���д�����������DNA��Ӿ�������ͼ2����1��2��3����Ʒ�Ļ����ͷֱ��ǣ���A��S��ʾ��صĻ���������
��֪�������鵰������A��ʾ����Mst II����ø�и��ɵõ�����Ϊ1.15kb��0.2kb������Ƭ�Σ�����0.2kb��Ƭ��ͨ�����������쳣���鵰������S��ʾ������ͻ��ǡ����Mst II����ø�и���ϣ����ʧȥ�˸�ø��λ�㣬��Mst II����ø������ֻ���γ�һ��1.35kb��DNAƬ�Σ���ͼl������Mst II����ø�Ա��Ϊ1��2��3��������Ʒ���д�����������DNA��Ӿ�������ͼ2����1��2��3����Ʒ�Ļ����ͷֱ��ǣ���A��S��ʾ��صĻ���������| A�� | AA��AS��SS | B�� | SS��AS��AA | C�� | AS��SS��AA | D�� | AS��AA��SS |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����

| A�� | �ٵIJ�����ȥ�� | B�� | �ڲ����˹��ڷ� | ||
| C�� | �ٺ͢ڵIJ�����ͬʱ���е� | D�� | �ڵIJ�����Ҫ�Դ����״� |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ������
 ��ͼ��ʾ����������Ũ�ȶԽ�ĸ��ϸ����CO2������Ӱ�죬���ͼ�ش�
��ͼ��ʾ����������Ũ�ȶԽ�ĸ��ϸ����CO2������Ӱ�죬���ͼ�ش��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com